Reports: DNI755563-DNI7: A Radical Approach to Conjugated Polymers
Emily Pentzer, PhD, Case Western Reserve University
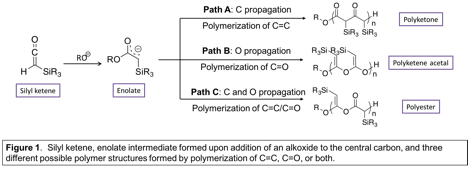
The polymerization of silyl ketenes using alkoxide initiators was explored by PI Pentzer and her lab. Specifically, triisopropyl silyl ketene (TIPS ketene) was exposed to the tertbutoxide anion at varying ratios and with different counter ions, and the reactions were monitored by Fourier transform infrared spectroscopy (FTIR), with products characterized by nuclear magnetic resonance (NMR) and size exclusion chromatography with multi-angle light scattering detection (SEC-MALS). TIPS ketene underwent polymerization to yield a novel polymer structure with ketone, acetal, and ester functionalities in the backbone. These results indicate that TIPS ketene can under polymerization of both the carbon-carbon (C=C) and carbon-oxygen (C=O) double bonds, illustrating an exciting new class of monomers and the polymers they can produce. An overview of the reactivity of silyl ketene with an alkoxide initiator is shown in Figure 1.
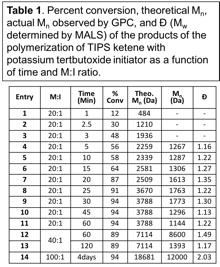
Initiation of TIPS ketene occurred by addition of tert-butoxide to the central carbon of the molecule to give an enolate intermediate. If an excess of tert-butoxide is used, this enolate could be quenched and the ester product isolated and characterized using standard techniques for small molecules. Alternatively, if an excess of TIPS ketene was used, propagation occurred by either the carbon or the oxygen atom of the enolate intermediate adding to the central carbon of another molecule of TIPS ketene. Polymerization only occurred at relatively high temperatures (above room temperature) and at high initial monomer concentration (5 M). If the oxygen atom propagates, the C=O of TIPS ketene polymerized and polyketene acetal was formed, and if the carbon atom propagates, the C=C of TIPS ketene polymerized and polyketone was formed; furthermore, if the carbon and oxygen atom alternatingly polymerize, both the C=C and C=O polymerized and polyester was formed. Under the conditions explored, all functional groups were formed on the same polymer backbone. Moreover, for all experiments the observed molecular weight (MW) of the product was lower than that expected based on monomer consumption, and at prolonged reaction times, MW of the product decreased (see Table 1). Therefore, secondary reactions, including backbiting and chain transfer occurred during polymerization and led to a non-controlled system.
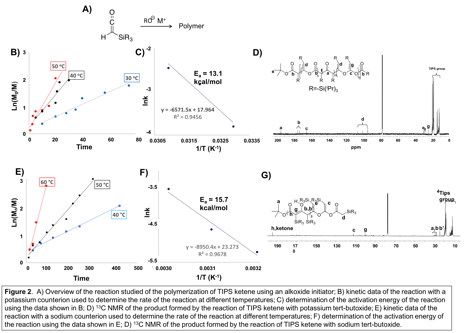
In addition to evaluating the polymerization of TIPS ketene, the impact of the cationic counterion on the polymerization and product formation was evaluated. Using a lithium counterion, only short chain oligomers were observed. However, with either a potassium or sodium counterion, polymer was formed, with the kinetics and products formed strongly impacted by the counterion identity. As seen in Figure 2B and 2C, by performing the polymerization of TIPS ketene with potassium tert-butoxide, at 30, 40, and 50 C, an activation energy of 13.1 kcal/mol is determined. Alternatively, by simply changing the counterion to sodium and running the reaction at 40, 50, and 60 C, the activation energy for the reaction is calculated to be 15.7 kcal/mol (Figure 2E and 2F). Characterization of the chemical composition of the products by carbon NMR indicates that in the presence of a potassium counterion, all functional groups are produced in an even ratio, while a sodium counterion led to a dominance of ketone functionality (compare Figure 2D and 2G).
The ultimate goal of this work is to access the controlled polymerization of silyl ketenes such that selective production of polyketone, polyketene acetal, or polyester can be realized without the disadvantageous secondary reactions of backbiting and chain transfer. Ongoing work focuses on using the identity of the counterion and solvent to control product identity and reaction rates, and exploration of different silyl substituents and initiators are also being evaluated.