Reports: UR453688-UR4: Transition State Analogues as Mechanistic Probes for Asymmetric Desymmetrization by Cinchona Alkaloid-Based Catalysts
Gretchen E. Hofmeister, Carleton College
Introduction
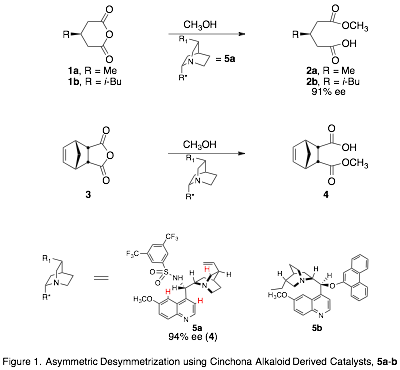
The goal of this project is to model the intermolecular forces governing enantioselectivity in the asymmetric desymmetrization (ASD) of cyclic anhydrides (Fig. 1).[1] This reaction introduces chirality into inexpensive achiral feedstocks, and has been applied in the total synthesis of biologically active compounds.1,[2] The model we develop can then be used to design or screen potential catalysts, in order to achieve high selectivity with a range of substrates.
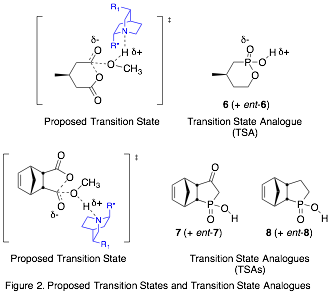
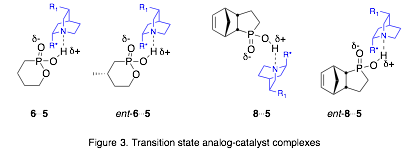
Our approach is to prepare transition state analogues (TSAs) for the organocatalytic ASD reaction and study the interactions between the TSAs and the catalysts, which are cinchona alkaloid derivatives.span>[3],[4] We are using tetrahedral phosphonic or phosphinic acids as TSAs to mimic the transition state and hydrogen bond to the catalyst (Fig. 2). To evaluate our model, we study the interactions of the TSA enantiomers with catalyst, measure the relative binding affinities of the enantiomers with catalysts, and correlate those with the enantiomeric excess (ee) of the ASD reaction (Fig. 3).
Goals
1. Synthesize TSAs and resolve into pure enantiomers.
2. Evaluate catalyst-TSA interactions by NMR spectroscopy and X-ray crystallography.
3. Measure the relative binding of two enantiomers of TSA to catalysts and compare with ee values, both experimentally and computationally.[5]
1. Synthesis
As described in previous reports, both R and S enantiomers of TSAs 6 and 8 have been prepared in 90% ee or greater, as well as the methyl ester of 7. Although the configurations of the enantiomers of 6 have been assigned, those of 8 have not.
2. Catalyst-TSA interactions
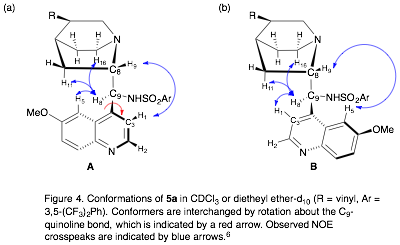
We have generally been unable to identify intermolecular NOEs between catalysts 5a or 5b and the TSAs 6 or 8. However, we have identified the catalyst conformer that dominates in the presence of TSAs 6 or 8. Catalyst 5a adopts two possible conformers in solution, which differ by rotation around the C9-aryl bond (Fig. 4). The conformer populations are equal in chloroform, but skewed towards conformer A in diethyl ether (A:B = 60:40).span>[6] A 1:1 solution of R-6:5a in diethyl ether-d10 shows the conformer populations continue to favor conformer A (A:B = 65:35). Similar results are observed in mixtures of rac-8:5a (A:B = 77:23 in CDCl3).
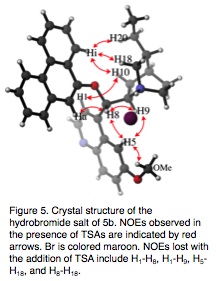
The conformation of catalyst 5b is also affected by the presence of TSAs. Analysis of the intramolecular NOEs between protons on catalyst 5b, in the presence of TSA 6 or 8, indicate that it adopts a gauche-open conformation. This is the same that we observe in the crystal structure of the HBr salt of 5b (Fig. 5). This conformer is consistent with the catalytically active conformation identified by Wong and Yang in computational studies,[7] but is inconsistent with experimental work by Deng and coworkers.[8]
3. Relative binding strength
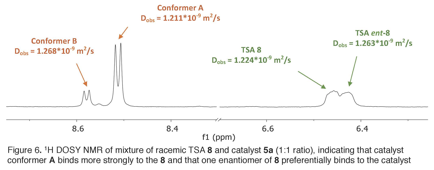
We have measured diffusion coefficients (D) for samples that combine catalysts 5a or 5b with racemic samples of 6 or 8 using DOSY NMR. For TSA 6, we have not observed a significant difference in D value for the two enantiomers with either catalyst. In contrast, the enantiomers of TSA 8 in the presence of catalyst 5a do have different D values (Fig. 6). Once we assign the absolute configuration of these enantiomers, we can interpret this result in the context of the ASD reaction.
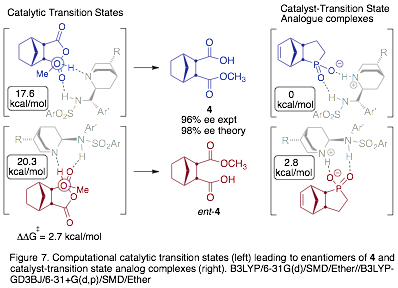
In previous work, we computed diastereomeric transition states (TSs) leading to the major and the minor enantiomers of 4 (Fig. 1).6 With PRF support, we now have computed the energies of the complexes between TSAs and catalyst 5a. Importantly, we find that the TSAs predict the same interactions and relative energies as the transition states (Fig. 7). However, the computational results indicate that TSAs bind more strongly to conformer B than conformer A, which is at odds with the preference calculated for the TSs and with our experimental results. We are currently working to address this discrepancy.
Impact on Students
A total of six students have done summer research supported by the ACS-PRF award (two for two consecutive summers). Their experience includes enantioselective synthesis and chiral resolution, multi-nuclear and two-dimensional NMR, and quantum computational chemistry. All students give a formal talk to the chemistry department, a poster presentation at Carleton, write a formal report, and continue research during the academic year. Four students have presented their results at a national ACS meeting (poster; one presented twice) and one presented a talk at the 29th Midwest Undergraduate Computational Chemistry Symposium. Two of the five graduates are enrolled in PhD programs (Northwestern U and U of IL, Urbana-Champaign), one is enrolled at the Mayo Clinic School of Medicine, and one is doing biomedical research at the U of MN, Twin Cities.
Impact on PI
The PRF award was excellent leverage for my successful NSF-MRI proposal (with two co-PIs) for a new NMR spectrometer. The project has also provided me with excellent experience in DOSY NMR and computational chemistry.
Endnotes
[1]. Borissov, A., et. al. Chem. Soc. Rev. 2016, 45, 5474-5540.
[2]. Diaz de Villegas, M. D., et. al. Chem. Soc. Rev. 2011, 40, 5564-5587.
[3]. Oh, S. H., et. al. Angew. Chem. Int. Ed. 2008, 47, 7872-7875.
[4]. Chen, Y.; Tian, S.-K.; Deng, L. J. Am. Chem. Soc. 2000, 122, 9542-9545.
[5]. Computational research was supported by PRF in summer 2017 because college construction prevented experimental work.
[6]. Blise, K.; Cvitkovic, M. W.; Gibbs, N. J.; Roberts, S. F.; Whitaker, R. M.; Hofmeister, G. E.; Kohen, D. J. Org. Chem. 2017, 82, 1347-1355.
[7]. Yang, H.; Wong, M. W. J. Am. Chem. Soc. 2013, 135, 5808-5818
[8]. Li, H., et. al. PNAS 2010, 107, 20625-20629