Reports: UR554214-UR5: Mechanistic Studies of Polyelectrolytes for Efficient Scale Inhibition
Xin Wen, California State University Los Angeles
The formation of sparingly soluble and insoluble inorganic salts (i.e., scale deposits) is a major problem in the petroleum industry operations. To avoid formation damage and well productivity reduction, it is crucial to remove scale deposits and control scale growth. The use of chemical scale inhibitors (i.e., antiscalants) is a common method for controlling scale deposits.
Commercial scale inhibitors for divalent cation carbonate and sulfate scales, such as calcium carbonate [(CaCO3) calcite and aragonite] and barium sulfate [(BaSO4) barite], include the polyelectrolytes that can dissociate phosphonates, carboxylates, and sulfonates anionic groups. However, it is imperative to identify highly efficient polymeric inhibitors and environmentally friendly ones to replace phosphonate inhibitors due to their environmental risks.
The efficiency of a polyelectrolyte inhibitor mainly depends on the properties of the charges, such as the strength, the number, the availability, and the conformation of the charges. Certain polypeptides with negatively and/or positively charged groups have been extracted from organisms and found to efficiently control the nucleation and crystallization of minerals (e.g., calcium carbonate). Their structures are attractive models for better understanding the inhibitor-mineral interactions and designing next generation antiscalants.
Antifreeze polypeptides (AFPs) from cold-adapted organisms (e.g., fish, insects, and plants) are known to inhibit the nucleation and crystallization of ice as well as certain non-ice like compounds. In fact, analogs of the alpha-helical type I AFP from winder flounder have been found to control the crystal growth of calcite.
In this project, we aim to correlate the charge and molecular properties of the polyelectrolytes with their efficiencies in inhibiting the scale crystal formation. In Year 3, we continued our investigation on the effects of charged residues on the surface of a natural polypeptide on calcium carbonate crystallization.
We have successfully demonstrated that the ASP containing polypeptides, DAFP and TmAFP, affect the crystallization of calcium carbonate. Here, we used the backbone of an AFP from the Tenebrio molitor beetle (Mealworm) (TmAFP) as a template to design an engineered TmAFP, referred to as D4. There are eight aspartic acids in D4 and seven of them are about equally dispersed on the surface of engineered polypeptide (Figure 1). Comparing to TmAFP, D4 extends the repeating pattern of ASP residues on the surface of the polypeptide. We thus expect that the effect of D4 on calcium carbonate crystallization would be more significant than that of TmAFP.
Figure 1. A tertiary structure of D4. The seven equally dispersed aspartic acid residues are shown in sticks.
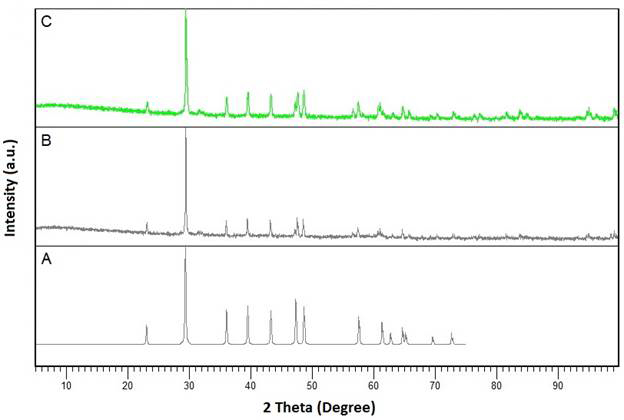
In Year 3, we characterized the achieved calcium carbonate (CaCO3) particles in the absence and presence of TmAFP using X-ray powder diffraction (XRD). The XRD patterns suggest that the achieved CaCO3 crystals in the absence and presence of TmAFP are calcite and the presence of the polypeptide does not change the form of CaCO3 crystals (Figure 2).
Figure 2. X-ray diffraction (XRD) patterns of calcite. (A) Theoretical XRD pattern for calcite. (B) The XRD pattern of achieved CaCO3 crystals in the absence of additives. (C) The XRD pattern of achieved CaCO3 crystals in the presence of TmAFP.
We also successfully prepared the engineered polypeptide, D4, and studied the formation CaCO3 of in the presence of D4 and TmAFP, respectively. The results for D4 and TmAFP were compared.
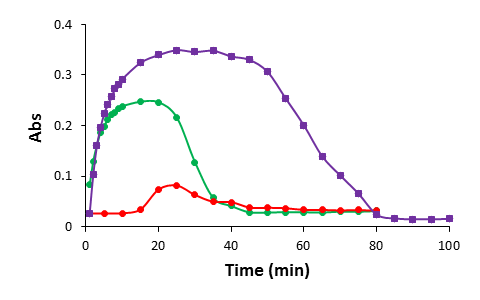
The characteristics of CaCO3 formation by mixing of equal volumes of equimolar sodium carbonate (Na2CO3) and calcium chloride (CaCl2) in the absence and the presence of D4 at room temperature. Variations in turbidity were monitored by apparent absorbance at 540 nm.
As expected, the presence of the engineered polypeptide, D4, significantly delays the induction time of calcium carbonate crystallization in the tens of nanomolar range and the effect is much stronger than that of TmAFP (Figure 3). The presence of D4 also significantly reduces the maximum absorbance (Figure 3), suggesting that the amounts of formed CaCO3 crystals are reduced.
Figure 3. The absorbance changes for calcium carbonate crystallization versus time: in the absence of additives (green dots) and in the presence of TmAFP (purple filled rectangles) and D4 (red dots), respectively.
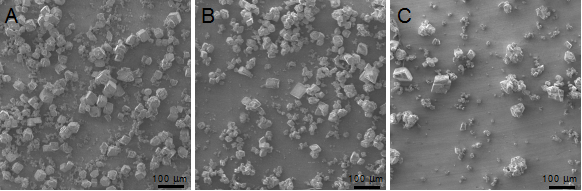
The scanning electron microscope (SEM) images were taken on the achieved CaCO3 solids using the ammonium carbonate diffusion method. Generally, fewer and smaller calcium carbonate solids were formed in the presence of D4 (Figure 4).
Figure 4. Scanning electron microscope (SEM) images of achieved CaCO3 crystals in the absence of additives (A), in the presence of TmAFP (B), and D4 (C), respectively.
These results suggest that the repeat polypeptides may serve as templates for highly efficient antiscalants.
This support enabled new discoveries in the field of scale inhibition by the PI and her students and exposed more interested students to experiencing chemical research. Almost all the supported students are underrepresented minorities. Such research experience has inspired these students to pursue STEM careers. The students’ activities and awards during the report period are summarized below.
Joshua Lugo (undergraduate) and Audrey Kishishita (undergraduate) won an outstanding presentation award and presented their research entitled, “Effects of Antifreeze Proteins on Calcium Carbonate Formation” at the 25th Annual Student Symposium on Research, Scholarship, and Creative Activities at Cal State LA.
Audrey Kishishita won the Travel Awards at the 2017 merging Researchers National (ERN) Conference in STEM and presented her talk entitled, “Effects of ice-binding proteins from cold-adapted insect Tenebrio molitor on calcite crystallization” at the conference in March. Audrey also presented her research paper at the 253rd ACS National Meeting in San Francisco, California, in April.
Jose Castellon (M.S. graduate student) won the Travel Awards at the 2017 merging Researchers National (ERN) Conference in STEM and presented his talk entitled, “Engineered antifreeze proteins to control calcium carbonate crystallization” at the conference in March.
Jose was also awarded NSF- Graduate Research Fellowship Program (GRFP) Fellowship on the effects of engineered polypeptides on calcium carbonate formation. Now he is a 1st year PhD candidate at the University of California, Los Angeles (UCLA).
The activities supported by this grant have great positive impacts on the students’ careers as well as on the career of the PI as a researcher, teacher, and mentor.