Reports: DNI1055033-DNI10: Discovery of Active Sites on Nanostructured Oxides for Desulfurization
Prashant Jain, PhD, University of Illinois, Urbana-Champaign
Summary and Impact: In the course of their functioning, solid-state materials such as adsorbents and sensors invariably go through reversible and irreversible structural reorganization. Atomic-level understanding of this structural dynamics is expected to be central to the rational design of materials that exhibit high efficiency, minimized dissipation, and long life-cycles. Although dynamical understanding has not been a cornerstone in the field, in-depth understanding is now enabled by the high spatiotemporal resolution of in-situ or dynamic transmission electron microscopy (TEM). In the past year, we employed dynamic TEM to spatially map, with atomic-resolution, the dynamics of structural phase transitions, in particular, in nanocrystals of the super-ionic solid, copper selenide. This solid has a unique lattice structure and ion transport characteristics on the nanoscale, findings we had made in the first year of the project. Our recent dynamic mapping studies revealed surface sites of the nanocrystal where the transition nucleates, the manner in which the phase transition progresses, and the strain changes in the anionic framework that accompanies the transition.
Background: My laboratory is deepening our atomic-level understanding of chemical transformations and phase transitions in the solid-state. One particular transition that serves as a model system involves a class of ionic solids called super-ionics. In a super-(cat)ionic material, the cations can transport through the solid with diffusivities (10-5-10-4 cm2 s-1) comparable to those in liquids or molten salts, resulting in exceptionally high ionic conductivities (> 10-3 𝛺-1 cm-1). The super-ionic phase is typically achieved at elevated temperatures via a phase transition. Below the phase transition temperature (Tc), the lattice structure exhibits an ordered arrangement of cations and anions. However, once the temperature is above Tc, the cationic sub-lattice becomes mobile, signifying a super-ionic phase.
Cu2Se is a classic earth abundant super-ionic solid. In each unit cell of Cu2Se, four Se2- anions, which have a face-centered cubic (fcc) arrangement with a lattice constant of 5.85 Å, form a rigid cage. The eight (or sometimes fewer) Cu+ cations are ideally located at the eight tetrahedral sites, forming an anti-fluorite structure. However, there are other crystallographic sites such as tetragonal, octahedral, and trigonal that the Cu+ ions can also occupy. At elevated temperatures, i.e., above ca. 400 K in the bulk, Cu+ cations can hop from an occupied site to other vacant sites, resulting in a disordered “liquid-like” sub-lattice, that typifies the high-temperature (HT) α-phase of Cu2Se. Below 400 K, the Cu+ ions in tetrahedral sites and empty tetrahedral sites form a ordered periodic lattice. Cu+ ion mobility is low in this low-temperature (LT) β-phase.
Even though the equilibrium ionic structure and properties of both phases of Cu2Se are well understood, the nature and kinetics of the phase transition remains mysterious. Therefore, we employed in-situ HRTEM imaging to watch with the atomic resolution a single Cu2Se nanocrystal undergoing the ordered-to-superionic transition. Such dynamical information is not attainable from an ensemble study because individual nanocrystals vary from one another in terms of the onset and progression of the transition. Averaging over the ensemble leads to loss of meaningful kinetic information.
Methods: Cu2Se nanocrystals were synthesized by a wet-injection method adapted from the procedure of Deka et al reported in 2010. The nanocrystals had a near-uniform morphology consisting of hexagonal nanoplates, 21.8 nm, on average, along their longest dimension. We used the electron beam (300 kV, 5 μA) to heat an individual nanocrystal and induce the super-ionic phase transition, which occurs in these plates at ca. 400 K, similar to the bulk, as per our digital scanning calorimetry (DSC) thermograms. Using fast, continuous 1-s time resolution HRTEM imaging on a Hitachi H-9500 instrument equipped with a Orius SC200, 2k x 2k pixel charge-coupled device (CCD) camera, we performed in-situ monitoring of the phase transition within single Cu2Se nanocrystals. Through acquired real-time videos, we were able to spatially map the phase transition with atomic resolution and also piece together the kinetics.
Results: The phase transition was monitored by means of a change in the lattice contrast pattern of the Cu2Se nanocrystal. In the LT vacancy-ordered phase, lattice fringes of 6.8 Å are observed along the <111> direction. This is because every fourth Cu+ layer along the <111> direction is vacant. The HT superionic structure has a disordered, effectively molten Cu+ sub-structure, resulting in a contrast pattern corresponding to the interplanar Se-Se spacing of 3.3 Å along the <111> direction.
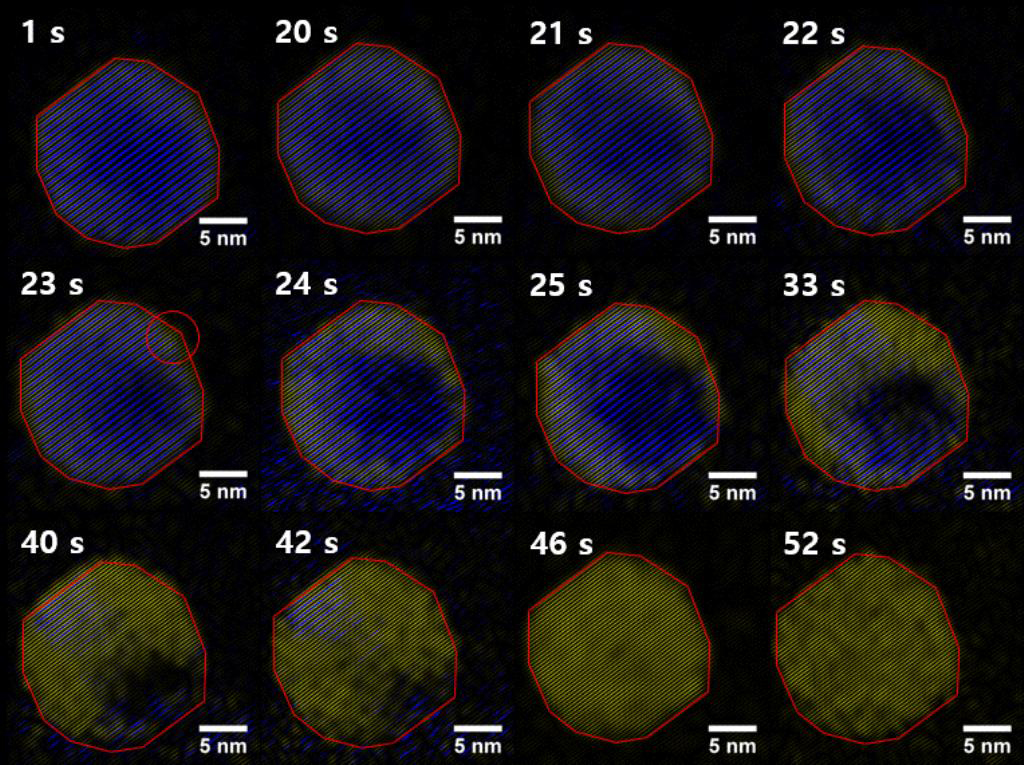 |
For imaging, individual nanocrystals exhibiting the LT phase were selected and subject to beam-induced heating, inducing conversion to the HT superionic structure. Select frames from continuous video imaging of one such nanocrystal undergoing the transition are shown in the figure below. This nanocrystal shows representative behavior across the sample set we studied, although a few nanocrystals show unique behavior that is not discussed here.
From the figure, one sees the initiation and progression of the phase transition (false-coloring in Image-J; blue regions correspond to the LT phase; yellow regions to the HT superionic phase). The nanocrystal undergoes little to no structural change until 20 s. At 23 s, the transition nucleates at a corner site on the nanocrystal surface (indicated by the circled region). Once the transition has initiated, the super-ionic phase grows across the nanocrystal along the direction parallel to {111} planes, until the entire nanocrystal is super-ionic (t = 46 s). We also note from our kinetic analysis that the accelerated growth of cation disordering is preceded by a compression of the Se cage. The latter is manifested by a decrease in the Se-Se <111> interplanar spacing from 3.4 Å to 3.3 Å, equivalent to a compressive strain of ca. 3%.
Conclusions: In conclusion, in-situ HRTEM imaging of the phase transition reveals deeper insights into the atomistic mechanism, information that will guide theorists modeling super-ionic phenomena and solid-solid transitions. In particular, we find a correlation between compressive strain and super-ionic disorder, which explains previous observations on nanosize effects on the phase transition temperature and establishes both grain size and lattice strain as handles for controlling ionic transport.











