Reports: ND953711-ND9: Characterizing Fuel Drop Vaporization at High-Pressure Environments
Song-Charng Kong, PhD, Iowa State University
This project is to conduct fundamental research on characterizing fuel drop vaporization at realistic combustion chamber environments. We first designed and fabricated a test chamber with proper piping, optical and data acquisition systems. After preliminary experiments to characterize fuel drop trajectory, the test chamber was further modified to sustain high pressures and temperatures. Various experiments of fuel drop vaporization were conducted. Data were processed using proper image processing techniques, which were also developed in this project.
Experimental apparatus and conditions
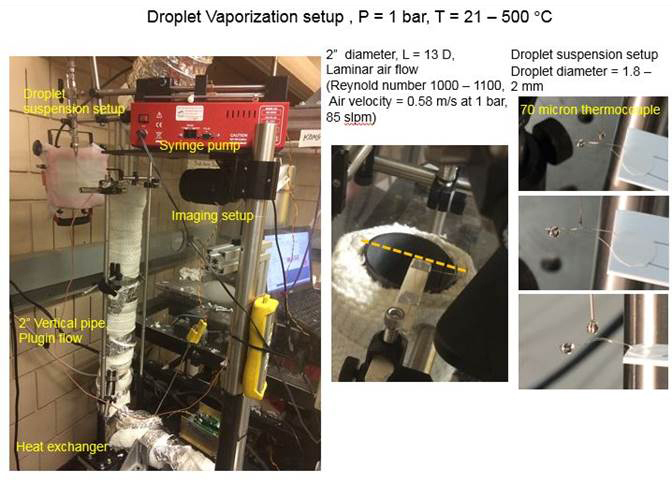
Experiments were conducted in a constant-volume chamber that can withstand high pressure and high temperature (as shown in Figure 1). The initial tests were under temperatures, ranging from 21 oC (294 K) to 550 oC (823 K) at 1 bar. Liquid n-heptane was used to test the repeatability of results at 100, 200, 300, 400, and 500 oC. Figure 1 also shows a fuel drop is generated and suspended at the tip of a micro fiber for vaporization study. An image processing technique was developed to quantify the drop size history for deriving the vaporization rate. Heated nitrogen was used to increase the ambient temperature for vaporizing the liquid drop. The velocity of the incoming nitrogen was measured for flow characterization. Both anemometer and PIV techniques were employed to obtain the velocity profile. The results have shown that the nitrogen flow velocity is relatively small and stays in the laminar regime, thus not enhancing heat transfer significantly.
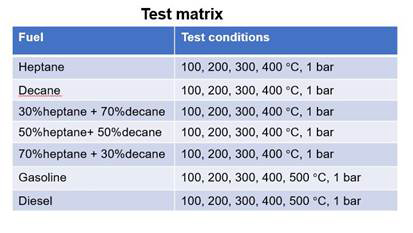
Some of the actual droplets images were shown in Fig. 2. As time goes by, the drop decreases in diameter because of vaporization. For example, at ambient temperature of 400 C, the liquid drop completely vanishes at 2.96 seconds. A parametric study was conducted for various test conditions, as shown in Figure 3. The fuels tested included single-component, bi-component, and practical multicomponent fuels. For single component fuel, results have shown the increase in the vaporization rate as the surrounding temperature was increased. The D-square curve reduces monotonically as the temperature increases.
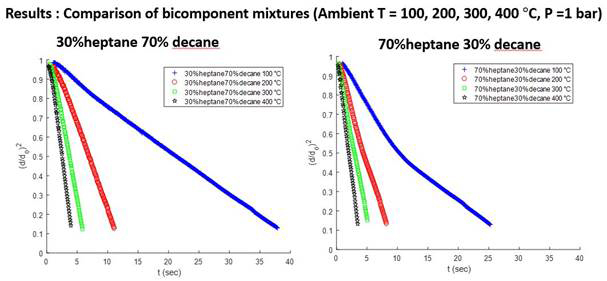
Examples of bi-component (n-heptane and n-decane) drop vaporization are shown in Figure 4. As the amount of decane increases, the vaporization rate decreases because of the higher molecular weight (heavier component) with n-decane. The different slopes of the D-square curve at different stages indicate the different vaporization rates of two components.
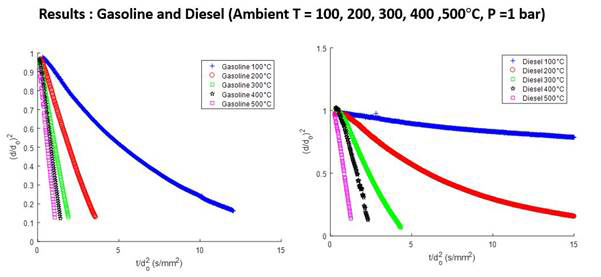
The D-squire curves of gasoline and diesel fuel drops show the nonlinear behaviors of the multicomponent drop vaporization for low and moderate ambient temperatures. At high ambient temperatures, the vaporization rate is high with a nearly linear D-square curve. Overall, gasoline has a relatively higher vaporization rate than diesel fuel, as anticipated.
Impact of the research
This research will create knowledge on fuel drop vaporization, which is an important process in a combustion process. Better understanding of the fuel vaporization process can enhance our ability to prepare the combustible mixture and help control the combustion process. The experimental data generated from this project will be used for validating computational models, which in turn can be used to predict the performance of combustion systems.
This research has allowed the PI to learn the characteristics of drop vaporization at a fundamental level. Experimental study of fuel drop vaporization is a new research area to the PI. This project also provided the opportunity for the graduate student to design, fabricate, and assemble a system for studying fuel drop vaporization. The graduate student has also conducted preliminary experiments to investigate the drop vaporization phenomena and gained in this area.
Figure 1
Figure 2
Figure 3
Figure 4