Reports: DNI1057072-DNI10: Facile Synthesis of Metal Organic Framework-Based Heterojunction Photocatalysts for CO2 Photoconversion into Hydrocarbon Fuels
Wei-Ning Wang, Virginia Commonwealth University
Objective
CO2 has been considered as one of the primary gases which cause the greenhouse effect. Various efforts have been devoted to controlling the CO2 emissions. On the other hand, CO2 is also an important precursor for chemical processes. The conversion of CO2 into value-added hydrocarbon fuels is promising to address the both issues of greenhouse effect and energy crisis. In the last funding period, we have carried out studies toward the facile synthesis of metal organic frameworks (MOFs)-based heterojunction photocatalysts for CO2 photoconversion into hydrocarbon fuels. Specifically, we have developed an aerosol method to synthesize the composites of MOFs and other functional materials. This method has been proven feasible for the fabrication of different composites of MOFs, which are promising for the capture and conversion of CO2.
Results
1. Rapid formation of MOFs based nanocomposites in microdroplets
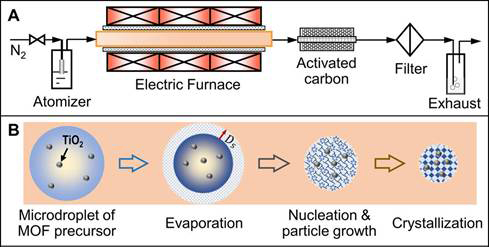
Our initial attempt focused on the fabrication of the composites of MOFs in microdroplets. Semiconductors are usually of photocatalytic activity. However, their low surface area and poor interaction with gas hinder the further improvement of CO2 photoconversion efficiency. On the other hand, MOFs, as an emerging class of porous polymer materials, have shown great potentials for the gas adsorption and storage. The composite of these two functional units is thus promising for an improved CO2 photoconversion efficiency. However, early efforts on the fabrication of the composites of semiconductors and MOFs usually require long synthetic time. To address this issue, we have developed an aerosol reactor as shown in Figure 1A, which is able to produce the composite of TiO2 and Cu3(BTC)2 (also known as HKUST-1) within seconds. Different from the conventional wet-chemistry method, where the synthesis of HKUST-1 usually takes long time (up to several days), our method produces microdroplets, which later serves as the microreactors. The small size of the microreactor enables the rapid assembly of copper precursor and organic linker, which is followed by the growth and crystallization of HKUST-1 (Figure 1B). It is also feasible to fabricate the composites of different MOFs and semiconductors. Microscopic heat and mass transfer within the droplets during the synthesis of MOFs have been calculated.
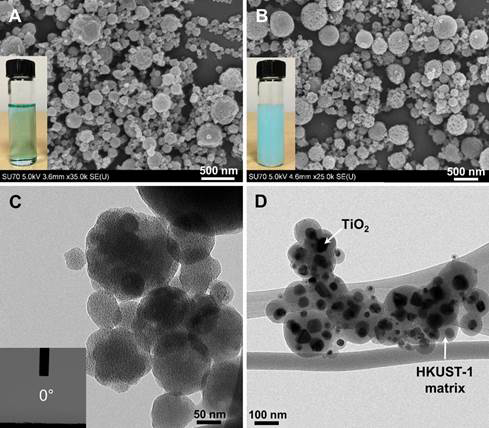
Figure 2 clearly shows the difference between pure HKUST-1 and HKUST-1/TiO2 composite. As shown in Figure 2D, all the TiO2 nanoparticles are well encapsulated within the HKUST-1. The photocatalytic reduction of CO2 was also carried with different photocatalyts. It was found that pure TiO2 only produced small amount of CO with a yield of 11.48 μmol/(g-TiO2·h) after being irradiated for 2.53 h. With the incorporation of HKUST-1, both the photoreaction rate and the CO yield were promoted. This promotion is amplified with increased amount of HKUST-1 in the composite. Specifically, with the ratio of HKUST-1/TiO2 increasing from 0 to 3.33, the peak time decreased from 2.53 to 0.78 h and the CO yield increased from 11.48 to 256.35 μmol/(g-TiO2·h), demonstrating more than 20-fold enhancement in CO2 photocatalytic reduction efficiency. The enhanced CO2 adsorption capacity of HKUST-1 was considered as a major reason, which was verified by diffuse reflectance infrared Fourier transform spectroscopy.
2. Facile synthesis of ZnO@ZIF-8 core-shell nanofibers
The nanofiber structure is also of great interest, considering its high aspect ratio and ability to avoid aggregation, which is important for many applications, including gas adsorption and catalysis. Only a few papers have reported the synthesis of metal oxide@MOF composites in the form of nanofibers thus far (e.g., Al2O3@MIL-53-NH2 and TiO2@UiO-66). The procedures for the fabrication of these metal oxide@MOF nanofibers in the reported papers are similar. In brief, atomic layer deposition (ALD) was employed to deposit metal oxide layers onto the polymer nanofibers. These metal oxide layers served as the template for the MOFs growth via the solvothermal method. However, the ALD deposition of metal oxide layers involves complicated and multi-step processes, which may limit the further exploration of this strategy.
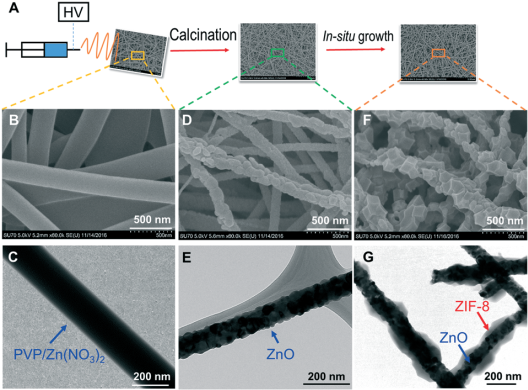
In order to simplify the synthesis procedure and avoid the use of costly equipment, we have developed a new strategy. Briefly, an electrospinning technique was used to produce polymer nanofibers containing zinc nitrate, which were then calcinated to obtain the ZnO nanofibers. These nanofibers subsequently acted as a sacrificial metal source and template for ZIF (zeolitic imidazolate framework) growth to form the ZnO@ZIF core–shell structure. This synthetic strategy and corresponding electron microscopy images are shown in Figure 3. This composite of ZnO nanofibers and ZIF-8 is also promising for the photocatalytic reduction of CO2.
Research Plan
In the second year of the project, research efforts will be dedicated to further expand the libraries of the composites of MOFs and other functional materials. With the incorporation of different functional units, various properties such as optical and magnetic will also be introduced. Further, the quantitative charge transfer within the MOFs-semiconductor hybrids during CO2 photoreduction will be unraveled by using advanced characterization tools, such as X-ray photoelectron spectroscopy, diffuse reflectance infrared Fourier transform spectroscopy, and ultrafast transient absorption spectroscopy.
Impact
The research supported by the ACS-PRF grant has resulted in three journal articles published/accepted in ACS Applied Materials & Interfaces, CrystEngCommon, and Nano-Micro Letters, respectively. The results were also disseminated in several national and international conferences. The project has trained one postdoc and one graduate student. This grant has served as an important seed funding for the PI to secure several other grants, such as a grant from the National Science Foundation and another one from the Virginia state.
Figure 1. Schematic illustration of (A) the aerosol reactor and (B) proposed HKUST-1 and HKUST-1/TiO2 formation steps inside a microdroplet.
Figure 2. SEM images of (A) as-synthesized HKUST-1 and (B) HKUST-1/TiO2; TEM images of (C) as-synthesized HKUST-1 and (D) HKUST-1/TiO2. (A, inset) and (B, inset) are the images of precursor solutions of HKUST-1 and 0.67 HKUST-1/TiO2, respectively. (C, inset) is the image of the contact angle measurement of HKUST-1 surface.
Figure 3. (A) Schematic illustration of the synthesis procedures; SEM images of (B) electrospun PVP/Zn(NO3)2 nanofibers, (D) ZnO nanofibers and (F) ZnO@ZIF-8 nanofibers; TEM images of (C) electrospun PVP/Zn(NO3)2 nanofibers, (E) ZnO nanofibers and (G) ZnO@ZIF-8 nanofibers.