Reports: DNI154422-DNI1: Cu-Catalyzed Vicinal Dicarbofunctionalization of Simple Alkenes
M. Kevin Brown, Ph.D., Indiana University
Overview: This PRF grant was used to advance Cu-catalyzed cross-coupling reactions, which is directly related to the proposed studies. Two directions were investigated over the last funding cycle.
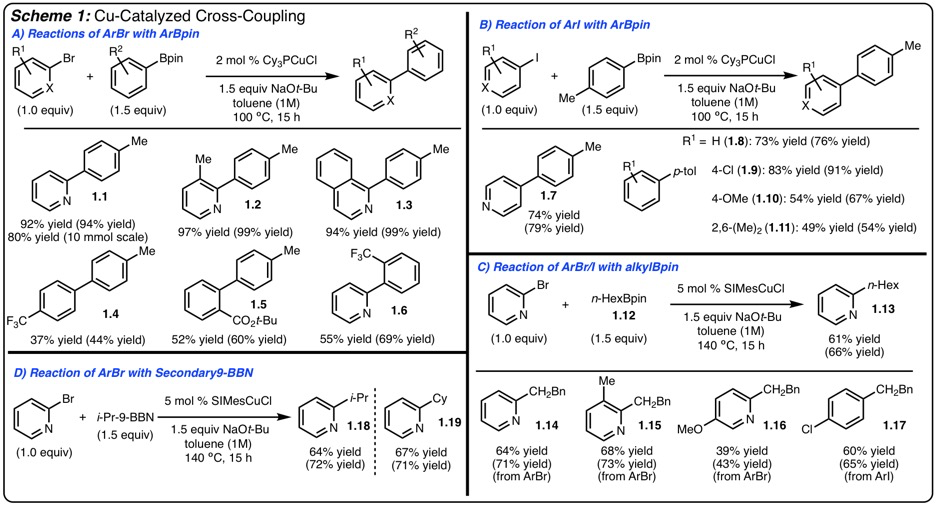
1) Cu-Catalyzed Cross Coupling of Organoboranes and Arylbromides:
In 2014 we reported a method for the cross-coupling of arylboronic esters with aryliodides promoted by ClCu-Xantphos. To extend the utility of these reactions, we sought to investigate reactions of arylbromides and to identify a less expensive catalyst. This initiative led to the finding that ClCu-PCy3 allowed for the cross coupling of heteroaryl and electron deficient arylbromides (Scheme 1A). In addition, we have shown that aryliodides also couple under these more practical conditions (higher concentration, fewer equivalents of base) (Scheme 1B).
Cross coupling reaction involving alkylboranes are significantly more challenging than use of arylboranes. Through change of the Cu-catalyst (ClCuPCy3 to SIMesCuCl) it was identified that coupling of not only primary alkylBpin (Scheme 1C) functioned well, but also use of secondary-9-BBN derivatives (Scheme 1D). In the case of secondary boranes, products arising from isomerization reaction were not formed.
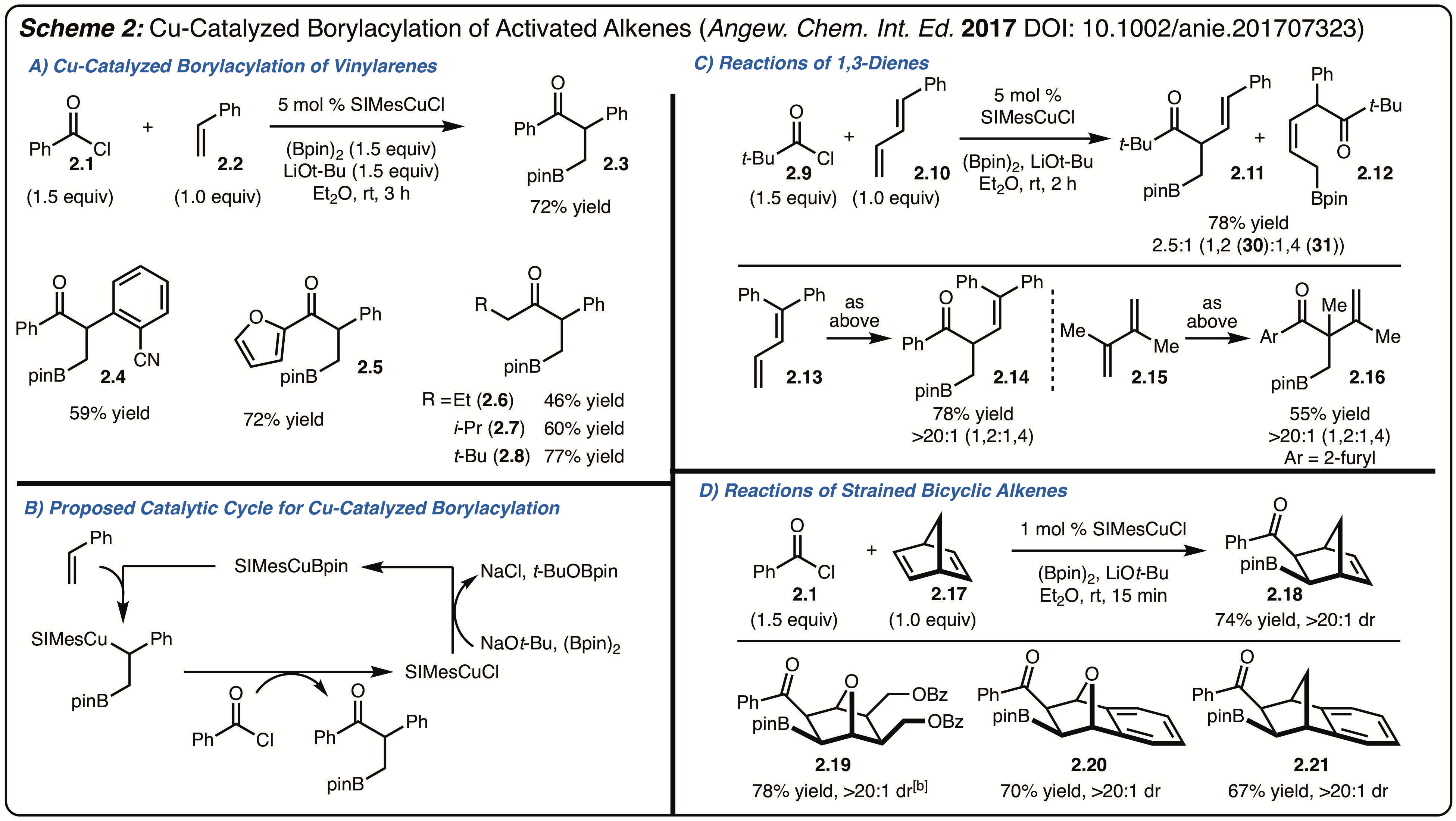
2) Borylacylation of Activated Alkenes.
In a related area of research we have investigated the Cu-catalyzed cross-coupling of acid chlorides (Scheme 1A). In these reactions a three-component process was developed that involves the coupling an in situ generated alkyl-Cu-intermediate generated by borylcupration of an alkene. Overall, these reactions led to the borylacylation of activated alkenes and likely function according the catalytic cycle outlined in Scheme 2B. A variety of alkenes were found to participate in these reactions such as vinyl arenes (Scheme 1A), 1,3-dienes (Scheme 1B) and strained alkenes (Scheme 1C). These reactions lead to the formation of synthetically useful product that would be difficult to prepare through alternative means.
Overview: Over the last three years during this award, a variety of Cu-catalyzed cross-coupling reaction have been developed. This has resulted in three published manuscripts with an additional paper that is to be submitted. These studies have also initiated the development of many other interesting directions within our lab. We sincerely thank the ACS-PRF for generous support of these projects.