Reports: DNI555519-DNI5: Nanostructured Catalysts for Tandem Catalysis Prepared by Atomic Layer Deposition
Xinhua Liang, PhD, Missouri University of Science and Technology
In the past year, this ACS PRF DNI-supported research has focused on the synthesis of metal nanoparticle catalysts by atomic layer deposition (ALD) for liquid phase reactions. Two catalysts were prepared and studied to assess their catalytic performance. One is highly dispersed platinum (Pt) nanoparticles (NPs) deposited on various substrates by ALD for the reaction of selective hydrogenation of citral to unsaturated alcohols, and the other is highly dispersed and highly stable supported gold-platinum (Au-Pt) bimetallic catalysts prepared using a two-step process for liquid phase reaction of glucose oxidation.
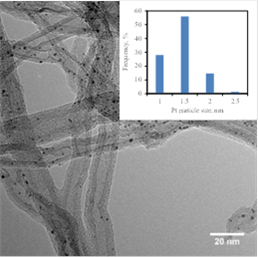
Selective hydrogenation of α,β-unsaturated aldehydes to corresponding allylic alcohols over metal-supported catalysts is attractive for both economic and scientific reasons. However, the hydrogenation of a carbonyl group is thermodynamically less favorable than that of a C=C double bond conjugate to the carbonyl present in citral, since the bond energy of C=C (615 KJ/mol) is lower than that of C=O (715 KJ/mol). It has been reported that catalytic performance (activity and selectivity) depends on many factors, such as type of metal and promoter. Among them, the nature of supports shows a critical influence on the activity of catalysts and product distribution during a selective hydrogenation reaction. In this study, Pt NPs deposited on different supports, were prepared by ALD in a fluidized bed reactor at 300 ºC to investigate and determine the influence of supports on the selective hydrogenation of citral to unsaturated alcohols (UA). Multi-walled carbon nanotubes (MWCNTs), silica gel (SiO2), commercial γ-alumina (γ-Al2O3), and porous ALD-Al2O3 particles, prepared by a template-directed synthesis approach, were used as the catalyst supports. TEM observation indicated that the diameters of Pt NPs were around 1.3 nm on all supports and Pt NPs were highly dispersed on the supports. Figure 1 shows a TEM image of Pt NPs on MWCNTs. The catalytic activity and selectivity of the Pt catalysts were evaluated by selective hydrogenation of citral to UA. Pt/SiO2 showed the highest activity, with 82% conversion of citral and a selectivity of 58%. Pt/MWCNTs showed the highest selectivity, with 65% selectivity to UA. The order of catalytic activity and selectivity to UA was observed to be Pt/SiO2 > Pt/ALD-Al2O3 > Pt/γ-Al2O3 > Pt/MWCNTs and Pt/MWCNTs > Pt/SiO2 > Pt/ALD-Al2O3 > Pt/γ-Al2O3, respectively. The acid-base property of supports was the dominating factor that determined the activity of all catalysts. On the other hand, the electronic effect and geometric structure influenced the product distribution of citral hydrogenation greatly for Pt/MWCNTs. The cycling experiments indicated that the Pt/ALD-Al2O3 had better stability than Pt/SiO2 due to the different interactions between Pt-SiO2 and Pt-Al2O3 in the solvent.
Figure 1. TEM image of Pt nanoparticles deposited on MWCNTs.
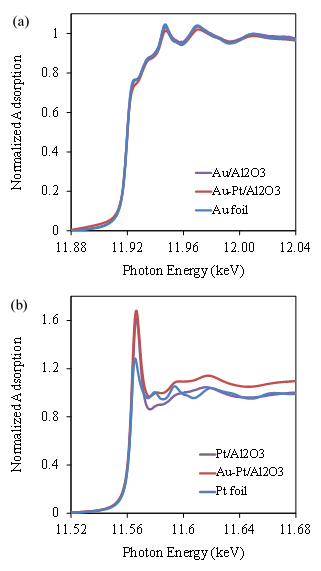
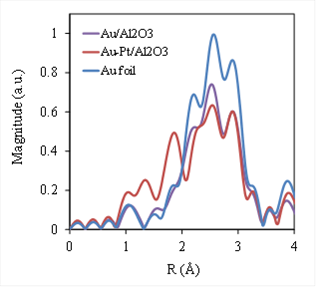
Bimetallic NPs have been widely investigated due to their unique properties and great potential in a variety of applications. Among them, gold (Au) – platinum (Pt) bimetallic NPs have attracted increasing interest due to their high activity in chemical reactions. However, leaching and the unstable problem of Au-Pt catalysts are barriers to keep their high activity for a long time. Metal nanoparticle catalysts prepared by using ALD are much more stable and recyclable due to the strong chemical bonding between the metal NPs and supports, which can prevent leaching in liquid phase reactions. In this study, we used a two-step approach that combined ALD and the conventional NaOH reduction method for preparation of Au-Pt bimetallic catalysts. Pt NPs were first deposited on γ-Al2O3 particles by ALD, and Au NPs were stabilized on ALD Pt/γ-Al2O3 particles from a hydrosol of gold clusters. Both TEM observation (Figure 2) and XAS analysis (Figures 3 and 4) verified the formation of Au-Pt bimetallic NPs with a narrow distribution centered at around 3.0 nm.
Figure 2. HRTEM image of 2.9 wt.% Au-6.4 wt.% Pt/Al2O3 NPs.
Figure 3. (a) Au LIII –edge XANES spectra (11.88-12.04 keV) of 2.9 wt.% Au/Al2O3 NPs, 2.9 wt.% Au-6.4 wt.% Pt/Al2O3 NPs, and Au foil; (b) Pt LIII-edge XANES spectra (11.52-11.68 keV) of 6.4% wt.% ALD Pt/Al2O3 NPs, 2.9 wt.% Au-6.4 wt.% Pt/Al2O3 NPs, and Pt foil.
Figure 4. Fourier transform of Au LIII edge EXAFS spectra of 2.9 wt.% Au/Al2O3 NPs, 2.9 wt.% Au-6.4 wt.% Pt/Al2O3 NPs, and Au foil.
ALD Pt/γ-Al2O3, Au/γ-Al2O3, and Au-Pt/γ-Al2O3 catalysts were used to study glucose oxidation to gluconic acid under identical reaction conditions. ALD Pt/γ-Al2O3 catalysts showed high activity and stability, as compared to a commercial Pt catalyst. Au-Pt bimetallic catalysts showed much higher activity and stability than the Au catalyst. The interaction between the Au-Pt and γ-Al2O3 support was stronger than that between Au and γ-Al2O3 support due to the anchoring effect of Pt on γ-Al2O3 support and the strong interaction between Au and Pt. The Au-Pt bimetallic catalysts synthesized by using the two-step method overcame the leaching problem and maintained high activity and stability. The strategy of combining ALD and a conventional method to fabricate a bimetallic catalyst with long-term stability provides an alternative way for preparing other highly dispersed, stable, and recyclable bimetallic catalysts.
In summary, this ACS-PRF grant has allowed us to complete the synthesis and testing of various metal nanoparticle catalysts by ALD. The findings and experiences resulting from this funding helped us develop worthwhile projects that have expanded the scope and significance of the initial project supported by ACS-PRF.