Reports: ND756400-ND7: Amine Containing Polymers: Efficient Access to a New Family of Functionalized Materials
Laurel L. Schafer, University of British Columbia
The atom economic and catalytic synthesis of amines has been targeted for decades. The Schafer group has contributed to these efforts by developing a regioselective intermolecular alkyne hydroamination catalyst (to make C-N bonds), as well as regioselective intermolecular alkene hydroaminoalkylation catalysts (to make C-C bonds). PRF has funded the exploration of using these catalytic methods to assemble previously unknown classes of amine containing materials. Amine containing polymers have a broad range of applications and here we summarize our new direction in the field of polymer chemistry and functional materials.
Catalytic Hydroamination to Access New Conjugated Amine Containing Polymers
Conjugated polymers like polyaniline and polyphenylene vinylene are important due to their wide application in non-metal conducting polymer, electronic devices, OLEDs and chemical sensors.i However traditional preparation of such materials can generate a significant amounts of hazardous waste. Here we show that, hydroamination can be used to assemble amine containing conjugated materials in a 100% atom economical reaction, using mild conditions (Figure 1).
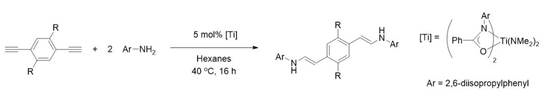 |
Figure 1
Furthermore, if diamines and dialkynes can be used, this would lead to conjugated polyenamines, a novel class of conjugated polymeric materials. Previously Greenberg and Stephen showed that this approach is possible but challenging due to regioselectivity problems with the hydroamination reaction.ii
Our group has developed a catalyst (Figure 1) for the regioselective synthesis of amine containing small molecules.iii In this project we found this catalyst to be powerful in the preparation of conjugated enamines. Such materials show interesting photophysical properties, including high quantum yields and large solventachromic shifts.
This synthetic strategy can also be extended to the synthesis of polymers with as little as 1 mol% catalyst loading (Figure 2). The polyenamine structure was confirmed by 1H-NMR spectroscopy. The Mn and Mw were measured by GPC.
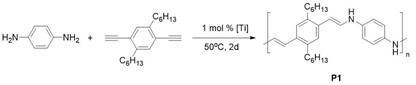 |
Figure 2
Various polymers with different diamine and dialkyne monomers were prepared. The quantum yield of these polymers can reach 0.43. Also, their emission spectrum covers the entire visible light region (Figure 3). These materials are being explored for light emitting and imaging applications.
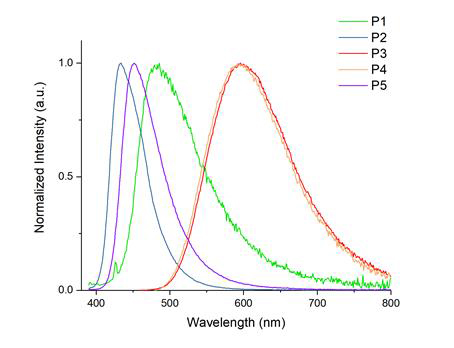 |
Figure 3 Normalized emission spectra of P1 – P5 in THF solution
Pendant Amine Containing Polymers By Hydroaminoalkylation
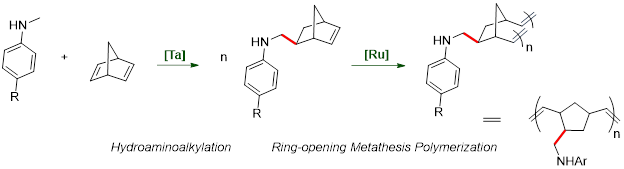
Hydroaminoalkylation is a viable route to access amine-containing monomers.iv Our group has developed tantalum-based catalyst systems for this atom-economic reaction. We can prepared functionalized monomers by hydroaminoalkylation to use in polymerization reactions by ring-opening metathesis polymerization (ROMP) (Figure 4).v,vi
Figure 4
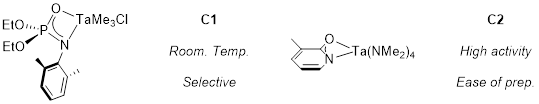
Two catalytic systems have been employed for the selective monoamination of norbornadiene (Figure 5):
Figure 5
The tantalum phosphoramidate catalyst C1 can do hydroaminoalkylation at room temperature.vii When used towards monomer synthesis, this complex can selectively functionalize one of the double bonds of norbornadiene to obtain functionalized monomers for ROMP. These reactions are performed ‘neat’ (solvent-free) and typically achieve yields up to 60% over 20 hours of reaction time. The tantalum pyridonate catalyst C2 has subsequently been developed and possesses a few notable advantages.viii This catalyst can be synthesized in one-step from commercially available starting materials, and is more robust than C1, with the capability to perform monomer synthesis on gram-scale in under 2 hours in moderate yields (60%).
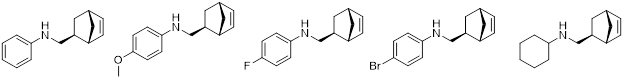
Through these catalytic systems, a variety of secondary amines have been explored (Figure 6).
Figure 6
The ability to polymerize these monomers is in contrast to other systems bearing polar functional groups which result in catalyst decomposition.ix,x Thus, a series of polymers with various secondary amines were obtained in high yield. These materials were anticipated to participate in variable hydrogen bonding, with the goal of tunable physical and mechanical properties. Therefore, aside from standard chemical characterization methods, the solution and melt rheology properties were investigated in an on-going collaboration with Professor Hatzikiriakos in the Chemical and Biological Engineering department at UBC. All the polymers studied were highly entangled and display dynamic crosslinking consistent with hydrogen bonding.
Conclusion:
Overall, we have developed efficient, atom-economic and catalytic methods to access a new families of amine-functionalized polymers. These protocols generate novel materials with advanced properties using atom-economic catalysis. This new materials are being explored for various applications.
i. a) Chen, D.; Miao, Y.-E.; Liu, T., ACS Appl. Mater. Interfaces 2013, 5, 1206-1212; b) Huang, C.; Barlow, S.; Marder, S. R., J. Org. Chem. 2011, 76, 2386-2407; c) Dhand, C.; Das, M.; Datta, M.; Malhotra, B. D., Biosens. Bioelectron. 2011, 26, 2811-2821.
ii. Greenberg, S.; Stephan, D. W., Poly. Chem. 2010, 1, 1332-1338.
iii. a) Lui, E. K. J.; Schafer, L. L., Adv. Synth. Catal. 2016, 358, 713-718; b) Lau, Y. Y.; Zhai, H.; Schafer, L. L., J. Org. Chem 2016, 81, 8696-8709; c) Yim, J. C. H.; Bexrud, J. A.; Ayinla, R. O.; Leitch, D. C.; Schafer, L. L., J. Org. Chem 2014, 79, 2015-2028; d) Borzenko, A.; Pajouhesh, H.; Morrison, J. L.; Tringham, E.; Snutch, T. P.; Schafer, L. L., Bioorg. Med. Chem. Lett. 2013, 23, 3257-3261; e) Zhai, H.; Borzenko, A.; Lau, Y. Y.; Ahn, S. H.; Schafer, L. L., Angew. Chem. Int. Ed. 2012, 51, 12219-12223; f) Zhang, Z.; Leitch, D. C.; Lu, M.; Patrick, B. O.; Schafer, L. L., Chem. Eur. J. 2007, 13, 2012-2022; g) Lee, A. V.; Schafer, L. L., Synlett, 2006, 18, 2973-2976.; h) Zhang, Z.; Schafer, L. L., Org. Lett. 2003, 5, 4733-4736.
iv. Lawrence, S. A., Amines: synthesis, properties and applications. Cambridge University Press: 2004.
v. Chong, E.; Garcia, P.; Schafer, L. L., Synthesis 2014, 46, 2884-2896.
vi. Sutthasupa, S.; Shiotsuki, M.; Sanda, F., Polymer journal 2010, 42, 905.
vii. a) Perry, M. R.; Ebrahimi, T.; Morgan, E.; Edwards, P. M.; Hatzikiriakos, S. G.; Schafer, L. L., Macromolecules 2016, 49, 4423-4430; b) Garcia, P.; Lau, Y. Y.; Perry, M. R.; Schafer, L. L., Angew. Chem. Int. Ed. 2013, 52, 9144-9148.
viii. Brandt, J. W.; Chong, E.; Schafer, L. L., ACS Catalysis 2017, 6323-6330.
ix. Lummiss, J. A.; McClennan, W. L.; McDonald, R.; Fogg, D. E., Organometallics 2014, 33, 6738-6741.
x. McClennan, W. L.; Rufh, S. A.; Lummiss, J. A.; Fogg, D. E., J. Am. Chem. Soc. 2016, 138, 14668-14677.