Reports: UNI154964-UNI1: Synthesis of Heterocycles by Palladium-Catalyzed [2+2+2] Cyclizations
Seann P. Mulcahy, PhD, Providence College
Transition metals play an increasingly leading role in synthetic organic methodology. When transition metals react with functional groups, the resulting coordination/organometallic complexes often create new opportunities for bond construction. By using these intermediates to one's advantage, organic chemists are able to perform transformations that were once costly, time-consuming, or generated large amounts of waste. Our group has been engaged in a research program that uses transition metal-catalyzed activation of triple bonds - alkynes and nitriles - to form pyridine-containing heterocycles. In this project, we hypothesized that palladium catalysts could be used to construct substituted pyridines via [2+2+2] cyclizations. Our preliminary work established an unprecedented, yet critical role for palladium in this reaction, and we are using ACS PRF funds to widen the substrate scope of this methodology at a primarily undergraduate institution. In 2015, we published our first paper using PRF funds (Organic Letters 2015, 17 (21), 5512-5514) on the synthesis of annulated pyrido[3,4-b]indoles using rhodium(I) catalysis, work which has expanded over the last year.
Our primary objective is to establish an undergraduate research program that facilitates heterocycle formation through palladium-catalyzed activation of alkyne and nitrile substrates. Towards this goal, we developed two specific aims, both of which received major emphasis during the second year of the funding period.
Aim 1: Develop a fully intramolecular [2+2+2] cyclization that will afford annulated pyridoindoles that are isomeric in structure.
Aim 2: Establish conditions for a partially intramolecular variant, which will yield annulated isomeric pyridines.
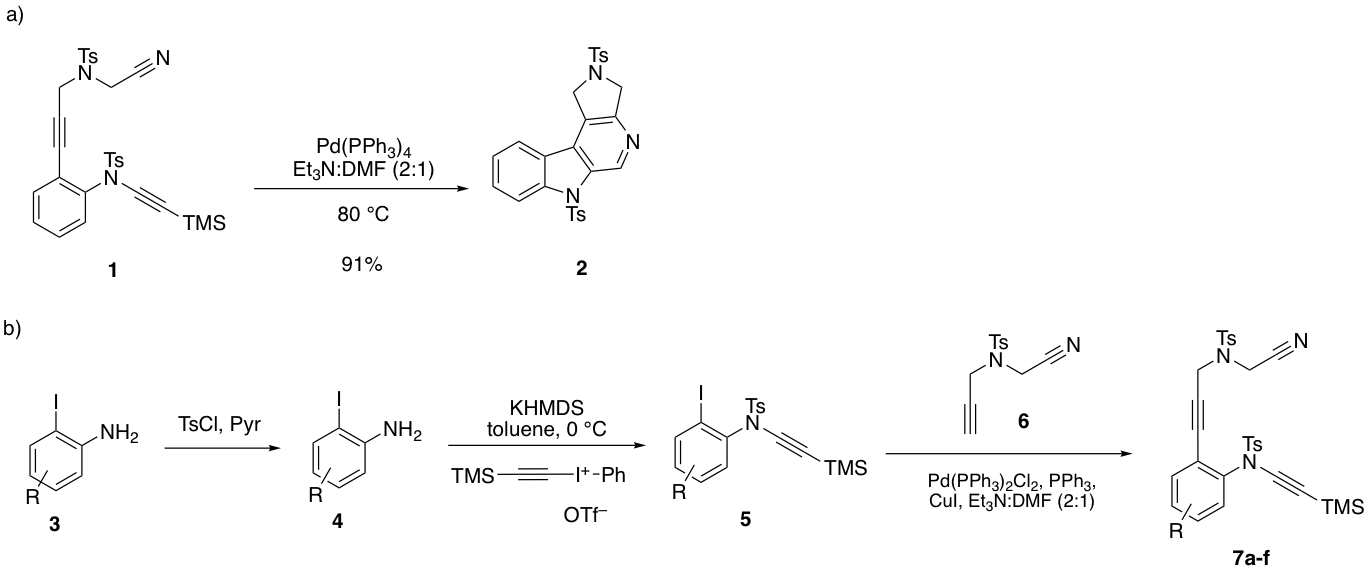
Preliminary work for Aim 1 established that the diynyl nitrile substrate 1 cyclizes rapidly to give the annulated pyrido[3,4-b]indole 2 in excellent yield (Figure 1a) in the presence of Pd(PPh3)4. Seeking to increase the substrate scope of this methodology, we used a three-step sequence to prepare diynyl nitrile substrates that bear electron-withdrawing and electron-donating groups on the benzene ring (Figure 1b). Over the past year, six substrates 7a-f (R = 3-Cl, 4-Cl, 4-OMe, 4-Me, 4-F, 4-CO2Me) have been prepared in moderate to excellent yields. With a no-cost extension of our ACS PRF Undergraduate New Investigator Award, we will be exploring the cyclization reactivity of 7a-f over the next year.
Figure 1. a) Palladium-catalyzed [2+2+2] cyclization of a diynyl nitrile; b) Preparation of differentially-substituted diynyl nitrile substrates
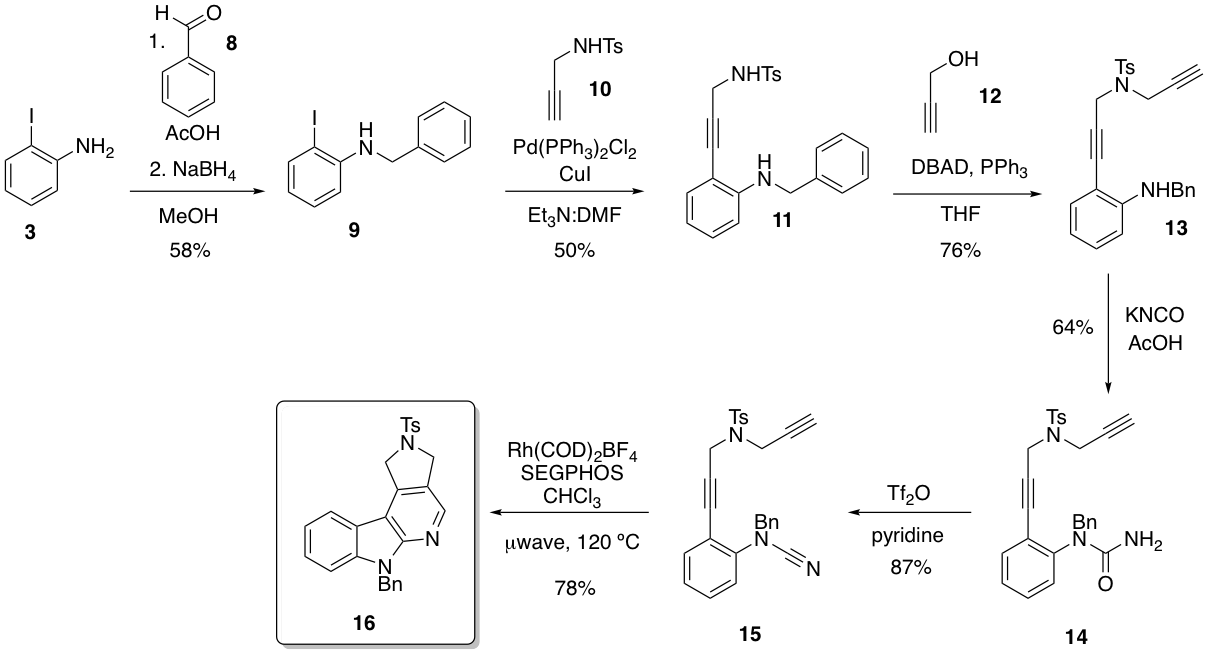
We recognized that establishing general conditions for a robust palladium-catalyzed [2+2+2] cyclization would not only be useful for the preparation of pyrido[3,4-b]indoles, but also for its isomeric cousin - the pyrido[2,3-b]indole. This would require transposition of a nitrogen and carbon atom in the diynyl nitrile substrate. Figure 2 shows the synthesis of pyrido[2,3-b]indole 16 in six steps. The final step in this sequence is a rhodium-catalyzed [2+2+2] cyclization involving a cyanamide functional group.
Figure 2. Synthesis of a pyrido[2,3-b]indole via rhodium catalysis
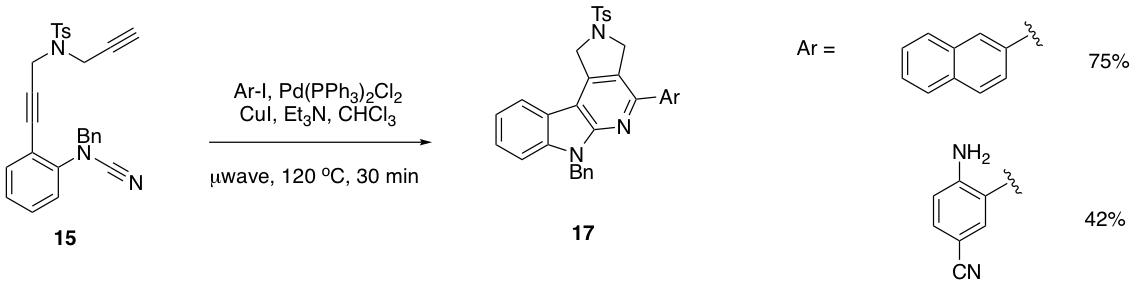
With preparation of substrate 15 in hand, we hypothesized that palladium could also be used for the final step. While using a cyanamide functional group for palladium-catalyzed [2+2+2] cyclizations has no precedent, we were especially intrigued by the potential for additional functionality to be introduced via a tandem Sonogashira reaction. Over the past summer, we have established conditions that effect this transformation in good yield, and will be optimizing the conditions and preparing a versatile substrate scope over the next year. So far, bulky aromatics, free amines, and a competing nitrile have little impact on the reaction's efficiency.
Figure 3. Palladium-catalyzed synthesis of a pyrido[2,3-b]indole
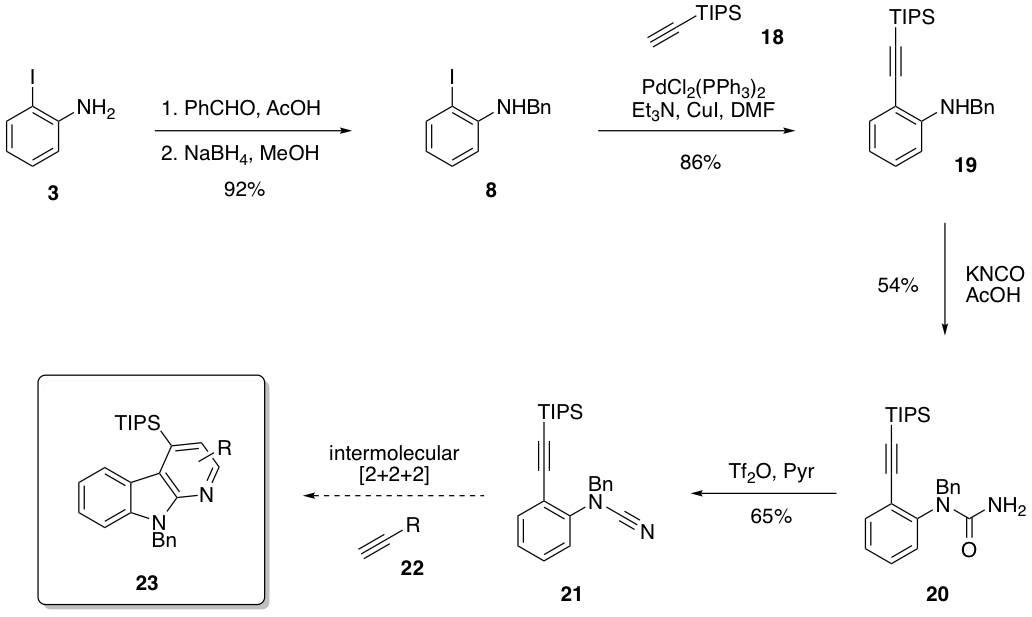
Finally, we have started to investigate a partially intermolecular variant of this methodology (Aim 2), which is entropically more challenging. Figure 4 shows the synthesis of the alkynyl nitrile substrate 21. While we intended to use a TIPS functional group as a protecting group for a free alkyne, the [2+2+2] cyclization reaction only yields trimerization of 22. We believe that the TIPS may be blocking the catalyst from accessing the pi bonds, so removal of the TIPS group prior to cyclization is a high priority. If successful, symmetrical and unsymmetrical alkynes can be used to create a substrate table, and the regioselectivity of this reaction can be explored.
Figure 4. Synthesis of a partially intermolecular [2+2+2] cyclization substrate
Since the length of the syntheses and the number of substrates investigated is time consuming in the context of an undergraduate institution, we have requested a no-cost extension until August 31, 2018. Remaining funds are still available for one undergraduate student, supplies, and travel, which will be sufficient to make significant progress on these projects. In the next year, we will finish our Hammett study of the palladium-catalyzed [2+2+2] cyclization of substrates 7a-f, prepare a substrate table of annulated pyrido[2,3-b]indoles via similar methodology, and further develop an intermolecular variant of this methodology. We expect that at least one manuscript will be submitted for publication in the next year.