Reports: UR155852-UR1: New Reactivity Mediated by Trimethylsilyl Trifluoromethanesulfonate
Chiles Wade Downey, University of Richmond
Introduction
The common goal of the projects described below is to discover new reactivity mediated by trimethylsilyl trifluoromethanesulfonate (TMSOTf) through its ability to react both as a Lewis acid and as a thermodynamic silylating agent. Over the past twelve months, significant progress has been made in the addition of nitriles to various electrophiles and the activation of propargyl carboxylates toward nucleophiles, as described below.
A. Addition of Nitriles to Acetals
Nitriles are common laboratory solvents and often function as masked carboxylic acid derivatives. The ability of (trimethylsilyl)acetonitrile (TMSACN) to act as a mild nucleophile in the presence of a nucleophilic catalyst has been adequately investigated, but we were more interested in the reactivity of a-silylnitriles under Lewis acidic catalysis. We recently discovered the ability of TMSACN to efficiently condense with dimethyl acetals. Over the past twelve months, we have completed our study in this field, which to our knowledge is unprecedented in the literature and is mediated by other common, mild Lewis acids.
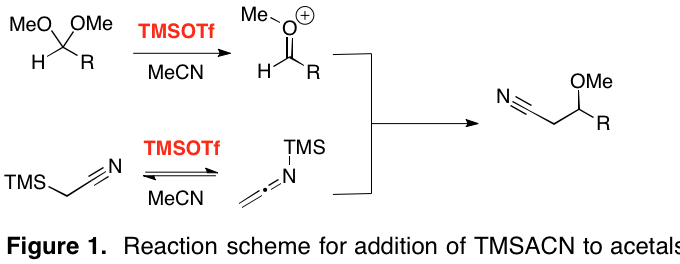
Our hypothetical mechanistic pathway is illustrated in Figure 1. The acetal is activated by TMSOTf to generate the reactive oxocarbenium ion. Simultaneously, TMSACN undergoes TMSOTf-catalyzed isomerization to its N-silylated tautomer. It is this isomerization where the reactivity of TMSOTf likely diverges from that of more traditional metal-based Lewis acids such as MgBr2·OEt2, ZnBr2, and BF3·OEt2, none of which appear to be capable of efficiently mediating the present reaction. Mukaiyama-like attack of the silylated nucleophile upon the oxocarbenium ion and desilylation yields the b-methoxynitrile. This speculative mechanism is our best explanation of the results at present.
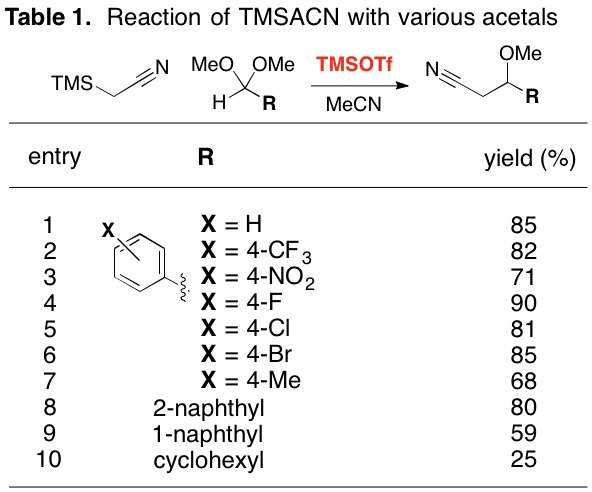
A variety of acetals have been reacted with TMSACN under similar conditions (Table 1). Most acetals derived from aromatic aldehydes react with good yields. Electron-poor substituents were especially successful. Relatively electron-rich substituents, however, showed themselves to be prone to elimination, yielding significant amounts of the a,b-unsaturated nitrile in addition to the b-methoxy product. These elimination reactions depressed the yield of the tolyl derivative (entry 7), and dominated the reactivity of the 4-anisaldehyde derivative (87% yield (85:15 trans:cis) of the acrylonitrile derivative, not shown). The cyclohexyl substrate shows that reaction is at least possible for aliphatic residues.
Outlook and Impact
This project was very recently published in Tetrahedron Letters. This family of projects has some importance for the career of the PI because it is the first example of TMSOTf-catalyzed reaction to appear from the group that does not require an amine base as co-catalyst. ACS-PRF-funded coauthor Jack Goodin has decided upon a career as a research scientist upon his graduation in 2018. Another student coauthor, Courtney Botelho, has graduated from the University of Richmond and begins a graduate program in Environmental Science at Wisconsin at the time of this writing. Also, student coauthor William Stith, a current senior of African-American descent, plans to attend graduate school in the 2018-2019 academic year. Followup projects will focus upon the replacement of acetals with the more useful aldehyde electrophiles, and the possibility of favoring a,b-unsaturated nitrile products. Sophomore Kylie Britt, whose career plans are currently flexible, began work on this project during the summer of 2017.
B. Reactivity of Propargyl Alcohols and Propargyl Carboxylates
The synthesis and reactivity of propargyl silanes is a major goal of our research group. One of our early attempts to synthesize propargyl silanes involved the activation of propargyl alcohols with silyl triflates. Although attempts to trap the resultant propargyl cations with silyl anions failed, ionization of the substrate was very successful under our conditions and the cation could be easily trapped by the simple hydride source triethylsilane (eq 1).
We speculated that this ionization method would be compatible with the in situ production of enol silanes from ketones using TMSOTf and an amine base. When the propargyl alcohol was added to the enol silane-formation reaction mixture, however, simple silylation of the hydroxyl group was the major product. Replacement of the alcohol with a propargyl acetate or propionate, however, gave rise to the desired alkylation pathway and yielded primarily the g-alkynyl ketone (eq 2). Minor amounts (typically 5-10%) of the allene product were sometimes observed.
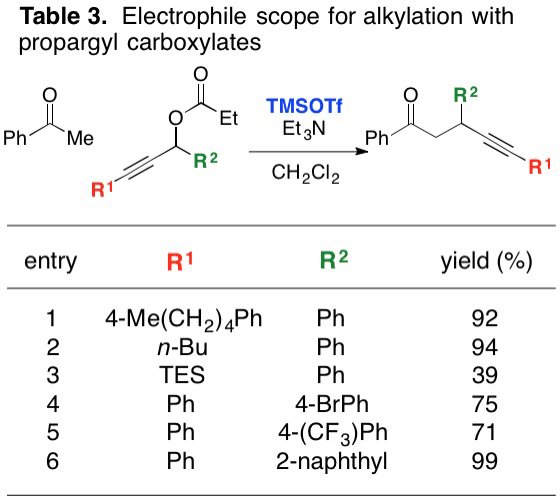
Optimization of the reaction across a range of ketones proceeded with good results (Table 2), and the reaction was recently expanded to include a thioester substrate. In general, more consistent results are observed if the acetate leaving group is replaced by a propionate, which slows the ionization step somewhat and helps to prevent rapid decomposition of the propargyl substrate. Recently, the reaction scope of the electrophile component has been our focus. Our current results on that front are illustrated in Table 3. These early results show a promising range of reactivity.
Outlook
Completion of the enol silane formation-alkylation component of this project is almost complete. Our short-term goal is complete the study of the reaction scope illustrated in Table 3. Furthermore, cyclization of similar products to yield furans is known in the literature, and we will make an effort to realize a one-pot reaction sequence where residual TMSOTf (or HOTf generated in situ) catalyzes the cyclization. This group of reactions should yield multiple publications and become the major focus of my group for the next few years. The student who primarily developed this project, Danielle Confair, graduated in May 2016 and attends graduate school in chemistry at Yale University, where she is a member of Jonathan Ellman's group. Three current students working on the project plan to enroll in chemistry graduate school: Xuechun Lin, Yiqi Liu, and Elizabeth Heafner. This grant provided Elizabeth Heafner's summer stipend during 2017. In addition, first-year student Dani Sklar began work on a related project during the summer of 2017, but her career plans are unformed at present.
Note: Another student, Grant Dixon, was funded by ACS-PRF during the summer of 2017 as well. His project is in its infancy, but he has discovered a reliable way for certain aldehydes to act as enolate precursors in the presence of TMSOTf and an amine base. Grant plans a career in the Navy following graduation.