Reports: DNI1055625-DNI10: Targeting Earth-Abundant Superhard Materials via Informatics and Optimized Chemical Bonding
Jakoah Brgoch, PhD, University of Houston
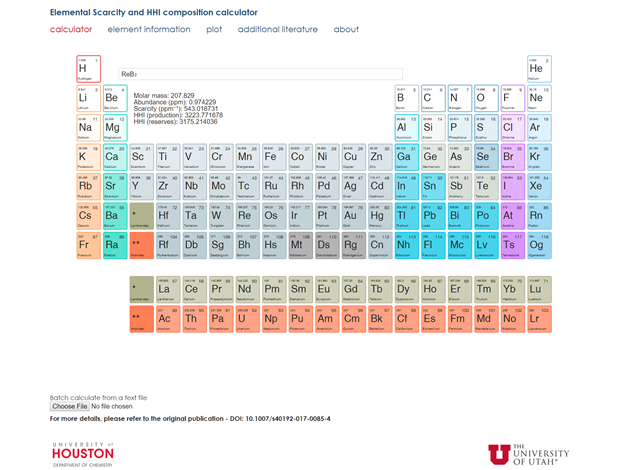
The research in the past year has focused on establishing and confirming materials design parameters that help direct our search for superhard materials. The specific focus involved examining characteristics associated with hardness such as crystal structure, composition, electron count, or average atomic volume. The results indicate that materials with high cohesive energy (as a measure of bond strength) and a high electron density tend to be the hardest compounds. We also developed metrics that addressed sustainability by determining a compound’s crustal abundance, supply and demand risk, and toxicity. For example, it is possible to use elemental accessibility and supply risk (crustal abundance, global production, reserves, use, etc.) to gauge the resource considerations ensuring all compositions are sustainable. One of the unique efforts in this area is our implementation of the Hirschman-Herfindahl index (HHI), which is commonly used in economics to measure the market concentration and supply risk of commodities/products. Recently, the HHI values (production, reserves) were determined for a majority of the periodic table, allowing us to compare the resource considerations for any elements of interest. Here, we calculate the HHI for compounds based upon the sum squared of market fraction for a given country, based on elemental production or reserves. To aid in the analysis of these sustainability metrics we produced a web-based resource (www.scarcitycalculator.com) for determining the resource parameters of any composition, as shown in Figure 1.
Figure 1. Screenshot highlighting the capability of scarcitycalculator.com determining the sustainability of superhard materials like ReB2
Using these structural design criteria and our online scarcity calculator, the project identified a potential superhard compound in the lanthanum-silicon-carbon-nitrogen system. La2Si5C6N has a comparatively abundant composition and is predicted to be a hard, refractory material. However, during the investigation of this compound, a structural instability was identified when calculating the ab initio phonon dispersion curves. The presence of imaginary modes in these calculations indicated the reported crystal structure (space group Pnma) is dynamically unstable with the eigenvectors showing a monoclinic distortion pathway leading to space group P21/c. We subsequently synthesized the phase using a high-temperature route and conducted a co-refinement with high-resolution synchrotron X-ray and neutron powder diffraction. The results showed a peak splitting in the diffraction patterns confirming the predicted lower symmetry crystal structure. Further, the combination of ab initio computation, neutron diffraction, and a neutron pair distribution function analysis supports that the anions are fully ordered and that carbon is only found on the central position of a star-shaped C(SiN3)4 unit present in this crystal structure. These results illustrate the power of combining computation and experiment to unequivocally solve crystal structures from polycrystalline powders. Our future research will continue to use computation to support all of our crystallographic results adding indispensable evidence for our crystal structure solutions.
Our research also investigated the chemistry of ultraincompressible, superhard materials based on systems containing heavy transition metals, which maximize the electron density, and light main group elements, which increase the cohesive energy. Rhenium subnitrides, Re2N and Re3N, are a perfect example of compounds that satisfy both criteria and yield high hardness (≈15 to 20 GPa), incompressible (≈400 GPa) materials. However, the volatility of nitrogen at the high temperatures required to synthesize these phases makes them prone to the formation of anion vacancies, which may alter the electronic structure and adversely affect the mechanical properties. Using first principle calculations, we investigated the possibility of vacancy formation in Re2N1–x (x = 0–0.25) and Re3N1–y (y = 0–0.25). The results of our research show that these phases are indeed prone to vacancies and the only way to prevent spontaneous vacancy formation is to apply external pressure (>5 GPa). However, if pressure is not applied and nitrogen vacancies form, there will be a significant deterioration of the shear modulus and the ultimate strength due to vacancy softening that arises from changes in the covalent bonding as probed through the crystal orbital Hamilton populations. These calculations show it is critical to control the applied pressure during synthesis to limit the concentration of vacancies and achieve the desired mechanical response.
Finally, during this past year the PRF funding supported multiple students, including Aria Mansouri Tehrani as a full time graduate research assistant, partly supported a post doctoral researcher, Anton Oliynyk, and two undergraduate chemistry majors at the University of Houston, Amber Lim and Gayatri Viswanathan (who was also supported by the University of Houston). Further, this funding helped support Zeshan Rizvi, who is a student at Houston Community College (HCC), to conduct research synthesizing new high hardness materials. Through the support of the ACS-PRF, Aria was able to present at a “machine learning in materials science and chemistry” workshop held at the American Chemical Society Florida Annual Meeting and Exposition (FAME) in Tampa, Florida. This activity provided training to a broad audience of chemists, physicists, and materials scientists who are interested in using machine learning and computation to predict a material’s properties.