Reports: ND754177-ND7: New Catalysts for Stereoselective Polymerization of Functional Alpha-Olefins
Lin Pu, University of Virginia
The research progress during the year of 2016-2017 is summarized below.
1. Study of Poly(NIPAM) via ATRP by Using a Chiral Initiator
Poly(NIPAM) represents the most extensively studied thermo-responsive polymer and has shown broad application in areas such as drug delivery, tissue engineering and biosensing. This polymer is soluble in both aqueous solution and common organic solvents such as methanol, acetone, THF, chloroform and methylene chloride. We have studied the use of a chiral initiator to synthesize poly(NIPAM).
Previously, the atom transfer polymerization (ATRP) of NIPAM in the presence of CuCl, tris[2-(dimethylamino)ethyl]amine (Me6TREN) and an initiator (methyl 2-chloropropionate) was used to generate the linear and narrow-dispersed poly(NIPAM)s. We have prepared a 1,1’-bi-2-naphthol (BINOL)-based initiator to conduct the ATRP of NIPAM. As shown in Scheme 1, the click cyclization of the BINOL-based dialdehyde (S)-2 with the azide 3 gave compound (S)-4. This compound was used as a diinitiator for the ATRP of NIPAM (5).
Scheme 1. Synthesis
of the initiator (S)-4 and polymer (S)-7.
Polymerization of NIPAM in the
presence of (S)-4, Me6TREN and CuCl was conducted in
isopropanol at room temperature. After 48 h, the resulting polymer (S)-7
was obtained and purified. This polymer was soluble in water as well as common
organic solvents such as methanol, THF, acetone, CH2Cl2 and
chloroform. The 1H NMR spectrum of (S)-7 in CDCl3
gave two signals at d 10.62 and 10.25 for the hydroxyl and
aldehyde protons respectively. The proton signal of the CH group connected to
the NH unit of the polymer was observed at d 4.00. The ratio of
the signal at d 10.25 versus that at d 4.00 is 1:98
which allows the molecular weight (Mn) of (S)-7 to be determined
as 22,900. Gel permeation chromatograph (GPC) analysis of (S)-7
in THF showed that this polymer had a quite narrow polydispersity (PDI =
1.20). The molecular weight determined by GPC relative to polystyrene
standards in THF is Mn = 4300 which is about 5 times smaller than that
determined by 1H NMR analysis. That is, using the polystyrene
standards greatly underestimates the molecular weight of this polymer. We also
prepared the BINOL enantiomer of (S)-7, (R)-7, from
the (R)-BINOL-based starting materials. Its molecular weight (Mn) is
23,000 as determined by 1H NMR analysis. GPC also showed a narrow
molecular weight distribution with PDI = 1.19.
The thermo-response of the aqueous
solution of (S)-7 was studied. When a water solution of (S)-7
(1.0 mM) was heated at a rate of 1 oC/min, it formed a cloud
precipitate at 32 oC. That is, this polymer-supported
BINOL-dialdehyde showed the same LCST as that of poly(NIPAM). The central
BINOL-dialdehyde unit did not change this unique property.
2.
The Use of Poly(NIPAM) 7 for Molecular Recognition We have studied
the fluorescence response of the polymer (R)- and (S)-7 in
the presence of amino acids. The water solubility of (S)-7
allowed us to examine the use of this polymer to recognize amino acids in
aqueous solution. We measured the fluorescence response of (S)-7
toward a chiral amino acid leucine (8), in HEPES or BICINE buffer
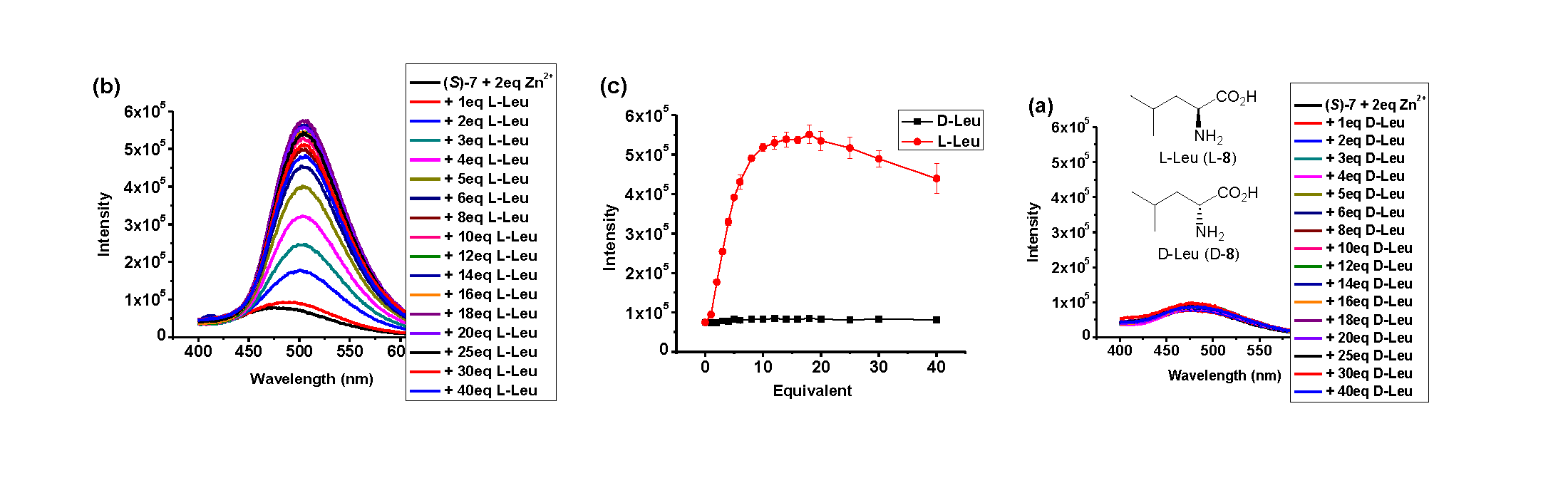
solutions at various pHs in the presence of Zn(OAc)2. It was found
that in BICINE buffer solutions (25 mM, pH = 8.80), (S)-7 showed
the highest enantioselective fluorescent response toward this amino acid and
thus this buffer solution was used for all the measurements in this work. As
shown in Figure 1, although D-8 generated little fluorescence response
of (S)-7, L-8 greatly enhanced the fluorescence at λem
= 504 nm. The fluorescence enhancement reached maximum when the concentration
of L-8 was greater than 10 equiv. At 10 equiv of 8, the
enantiomeric fluorescence enhancement ratio [ef = (IL
- I0)/(ID - I0)] is 45.1
which represents a very high enantioselectivity.
We
also examined the use of the enantiomeric sensor (R)-7 to
interact with the amino acid under the same conditions. It was found that D-8
greatly enhanced the fluorescence of (R)-7 but L-8 did
not. That is, the fluorescence responses of (R)-7 toward the
amino acid are the mirror images of those of (S)-7. This
confirms the observed high enantioselectivity of the fluorescent sensor. It
also demonstrates that the random steric structure of the poly(NIPAM) chain
does not interfere with the chiral recognition of the BINOL core.
Figure 1.
Fluorescence titration of (S)-7 (1.0 mM, 1 equiv) with (a) D-8
(4.0 mM) and (b) L-8 (4.0 mM) in BICINE in the presence of Zn(OAc)2
(2.0 mM in water, 2 equiv) (Reaction time: 2 h, then diluted 100 fold with
BICINE. λexc = 320 nm. Slit: 5/5 nm). (c) Fluorescent
intensity at λem = 504 nm versus the equivalency of D- and L-8
from three independent measurements.
3.
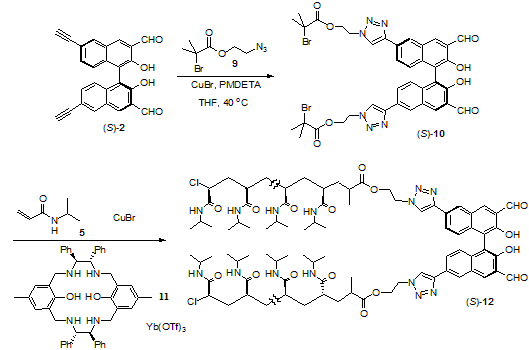
Stereoselective Polymerization by Using a Macrocyclic Catalyst In order to conduct the
stereoselective polymerization of NIPAM in the presence of the BINOL-based
initiator, we have synthesized the tertiary bromide-derived initiator (S)-10
from the reaction of (S)-2 with the azide 9 (Scheme
2). Polymerization of NIPAM in the presence of the previously prepared
macrocyclic catalyst, that is the combination of ligand 11 and Yb(OTf)3,
has been conducted which generated the predominately isotactic polymer (S)-12.
NMR study shows that (S)-12 contains 87% (m) isotactic structure
unlike the sterically irregular polymer (S)-7. The isotactic (S)-12
has much lower solubility in water and organic solvent. The application of (S)-12
will be investigated.
Scheme 2. Stereoselective
Polymerization in the Presence of a Macrocylic Catalyst.
3.
Summary We have conducted the ATRP
of NIPAM by using a BINOL-dialdehyde-based initiator. Application of the
polymers in the fluorescent recognition of amino acids has been investigated in
both water and organic solvents. Using a chiral macrocyclic compound in
combination with a Lewis acid complex, we have also prepared a highly isotactic
poly(NIPAM).