Reports: DNI156505-DNI1: Chiral alpha, alpha - Disubstituted Amines via the Allylic Azide Rearrangement
Joseph Topczewski, PhD, University of Minnesota
Research in the Topczewski lab is focused on developing new synthetic methods to enable efficient and sustainable chemical syntheses. The funded work aims to develop more efficient synthetic methods for the synthesis of sterically congested chiral amines. To accomplish this goal, we proposed developing methods to resolve allylic azides, which are in equilibrium. Key to this proposal was identifying reagents and pathways that could selectively functionalize the allylic azide’s alkene.
During the last funding period (2016-2017), we made significant progress towards these goals. This is best reflected in the initial series of publications from my group. The progress made will be described in the context of each of our initial publications that acknowledge the ACS-PRF.
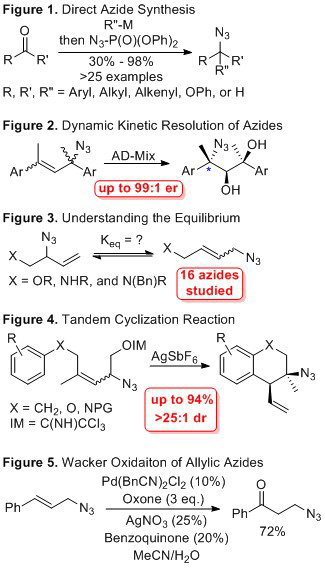
Publication 1: The first challenge to executing the proposed research was developing an efficient synthesis of allylic azides. While many methods are known for azide synthesis, most methods typically require multiple steps or utilize reactive intermediates, which can be difficult to isolate or purify. To circumvent these issues, we developed a direct synthesis of allylic azides. Our approach is based on the addition of an organometallic reagent (organolithium or Grignard reagent) to an aldehyde or ketone. The resulting alkoxide was trapped in situ with diphenyl phosphoryl azide. This resulted in a direct synthesis of these azides after subsequent nucleophilic attack with azide anion, which was also liberated in situ. We demonstrated that this “one pot” method successfully generates >25 azides. A graphical abstract of the published work is shown in Figure 1 (Eur. J. Org. Chem. 2016, 2016, 4805, DOI: 10.1002/ejoc.201600856). We are now using this method to advance the research described below.
Publication 2: With these azides in hand, we were able to look into the core hypothesis of this proposal. We proposed that the allylic azide rearrangement could be used as the racemization pathway in a dynamic kinetic resolution. To answer this question, we started with symmetric allylic azides. We investigated various methods for alkene functionalization. One classical alkene functionalization is the Sharpless asymmetric dihydroxylaiton. After optimization, we were able to use the Sharpless assymetric dihydroxylation in conjunction with the allylic azide rearrangement in a dynamic kinetic resolution. We obtained good yields, high dr, and extraordinary er (up to 99:1). A graphical abstract from this work is shown in Figure 2 (J. Am. Chem. Soc. 2017, 139, 7737. DOI: 10.1021/jacs.7b04203). The results are proof-of-concept that the core of this proposal is possible and were able to advance the products of this reaction to valuable building blocks, such as amino acids.
Publication 3: To expand our proof-of-concept data to un-symmetrical systems, we needed to gain a fundamental understanding of the physical constants that govern the allylic azide rearrangement. To do so, we systematically varied the substituents on the allylic terminus. As part of this study, we synthesized 16 unique allylic azides with varied groups. The equilibrium constants of these 16 azides was measured experimentally in four solvents. We observed no solvent effect, but we were able to correlate the observed equilibrium constant with pKa for a series of -OR derivatives. Two series of allylic amine derivatives were synthesized and varied by being either –N(H)R or –N(Bn)R derivatives. By comparing this series of derivatives in 4 solvents, we were able to conclude that hydrogen bonding is not likely a dominant feature of these compounds. This contradicts a previous hypothesis that was present in the literature and provides a more basic understanding of these systems. Calculations by DFT led to the conclusion that stereoelectronic effects are likely the dominant contributor to the equilibrium constant. A graphical abstract from this work is shown in Figure 3 (Eur. J. Org. Chem. 2017, 2017, ASAP. DOI: 10.1002/ejoc.201700693). This work provides a basis for our continued focus on gaining a mechanistic understanding of this rearrangement. This work will be featured on a forthcoming journal cover from Eur JOC.
Emerging Work: We submitted our fourth manuscript that is connected to this grant (Figure 4). It features a distinguished carbon-carbon bond forming reaction. This reaction forms a variety of building blocks through resolution of the mixture of allylic azides. This process uses several different reactivity principles that we identified to control a Friedel-Crafts cyclization. The starting material contains four different azide isomers and from this mixture we can obtain high yields (up to 94%) and excellent selectivity (>25:1). This is extraordinary given that in some cases the reactive isomer is only a trace component of the mixture. We have >20 examples of this process and these products have unique functionality that can be used in target directed synthesis. We used our method in a total synthesis of hasubanan to illustrate this point
Continuing Work: We have several other aspects of this project in various levels of development. These include developing a Wacker oxidation of allylic azides, triggering a Stieglitz rearrangement of our azides, and developing a fundamental mechanistic understanding of this process that includes racemization pathways. The Wacker oxidation was one of the aims originally proposed. We have encouraging preliminary data and have optimized the oxidation of cinnamyl azide to provide the acetophenone derivative in >70% isolated yield using oxone as the terminal oxidant (Figure 5). We are expanding this reaction to other cinnamyl azides. We are also expanding this reaction to other allylic azides that equilibrate.
Conclusion: We have made significant progress towards harnessing the allylic azide rearrangement. Over the last funding period, we published 3 paper and have one more submitted. We have several promising projects in progress to expand these preliminary results. We provided proof-of-concept validation for the core aim of this work and we are vigorously pursuing means to generalize this strategy.