Reports: UNI155657-UNI1: Tunable Triarylmethyl Carbocation Catalysis
Cheyenne S. Brindle, PhD, Trinity College
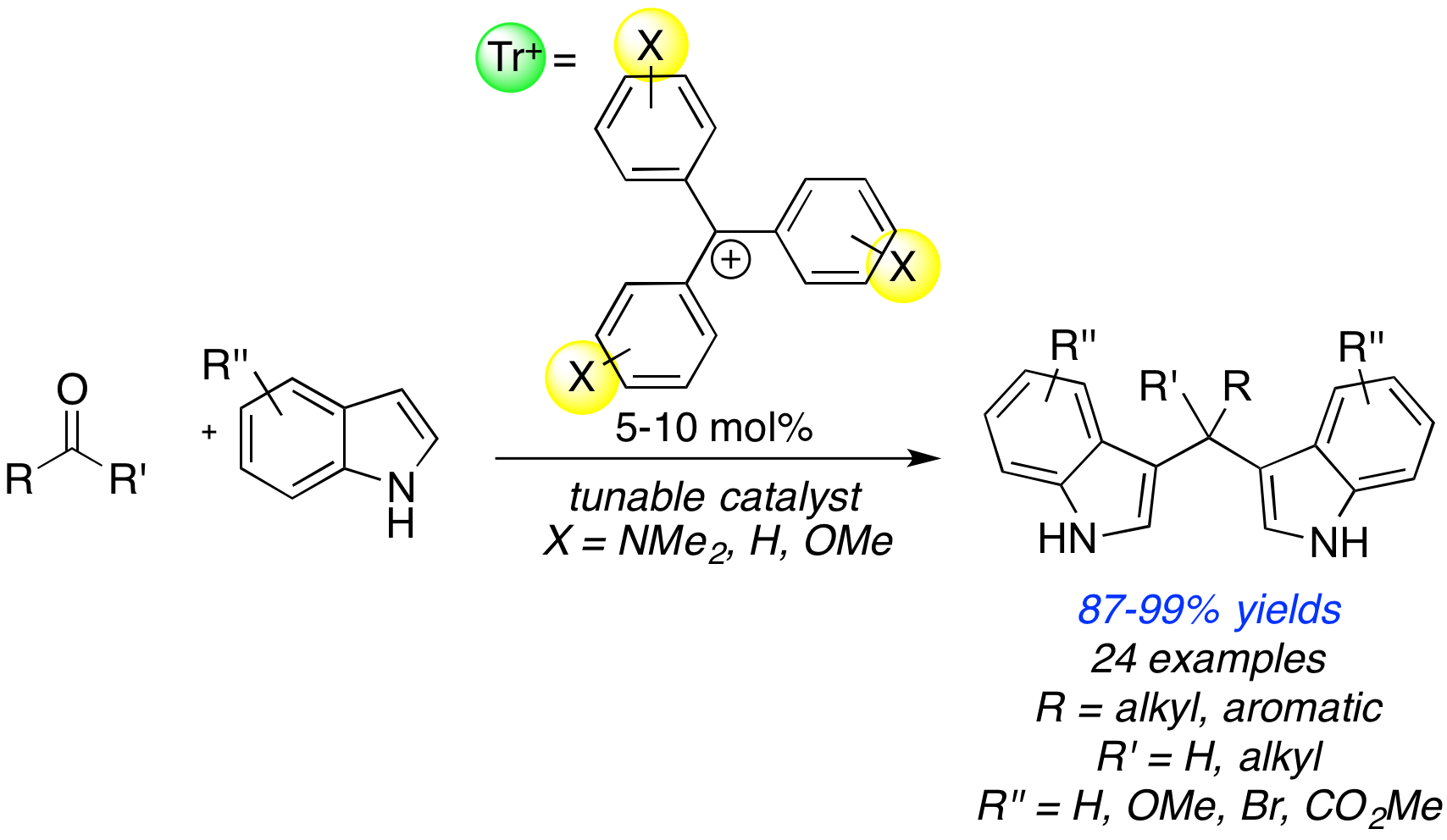
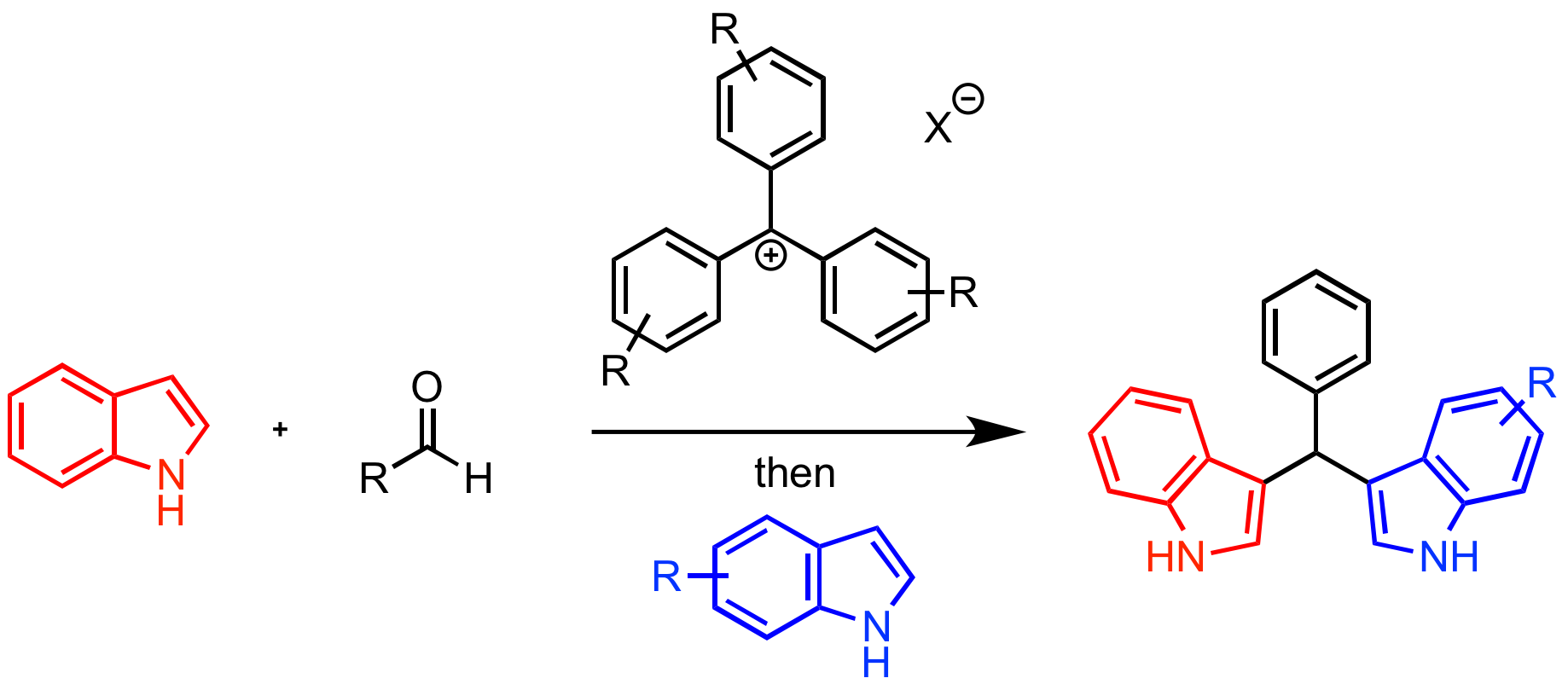
In the second year of my Petroleum Research Foundation Grant I was able to complete the first reaction that I had identified as a suitable reaction for triarylmethyl (trityl) cation catalysis, begun in my first year. The reaction parameters were optimized by my research student, Nicholas Boekell, for model substrates indole and benzaldehyde in the first year of this project. The substrate scope was completed this year by myself and my research student Wilfried Nganyak Tentchou. Less reactive substrates required catalyst tuning to enhance reactivity, which was done jointly by myself and first year student Maria Boucher. We were able to increase the reactivity of the trityl cation by changing the substituents on the aromatic rings of the trityl cation, resulting in enhanced reactivity for more sterically-hindered, or electron-rich substrates. We used the library synthesized last year by Jordan Reid to find a suitably reactive trityl cation catalyst. This highlights the utility of these cations, since their remarkable tunability allows for an extremely wide range of activities. This work has been accepted to SynOpen, the new open source journal connected to SynLett and Synthesis. The usual fee for publication was waved for our paper by the editor.
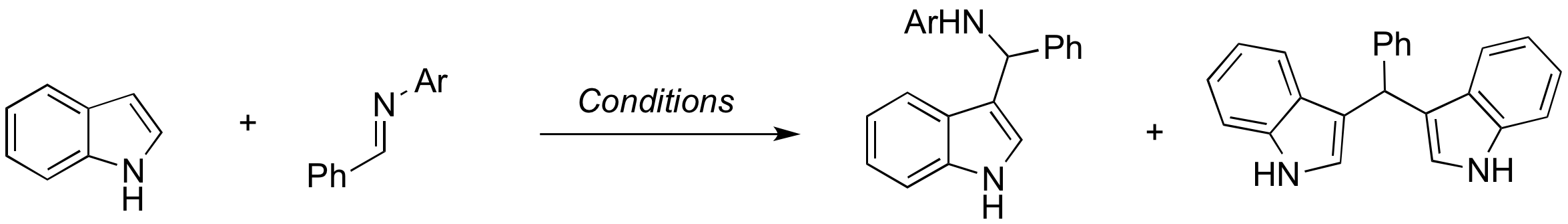
Last year, I began exploring whether it was possible to get a single addition product, rather than the double addition product from the reaction of indole with aldehydes. This was achieved by exchanging the aldehyde for an imine, which gave good single addition selectivity after optimization. We experienced difficulty with imine hydrolysis. Melissa Guarino-Hotz painstakingly investigated this problem, and eventually found that our new shipment of deuterated chloroform was causing hydrolysis. With this problem solved, Vanessa Jones, Matt Epstein, Kevin Bardelski, and myself worked over the summer to synthesize imines, optimize the first step, and work on the one-pot reaction. We have finished the optimization and substrate scope investigation for the first step. What remains to be done before publication is characterization of some of the products, and completion of the substrate scope for the one-pot reaction.
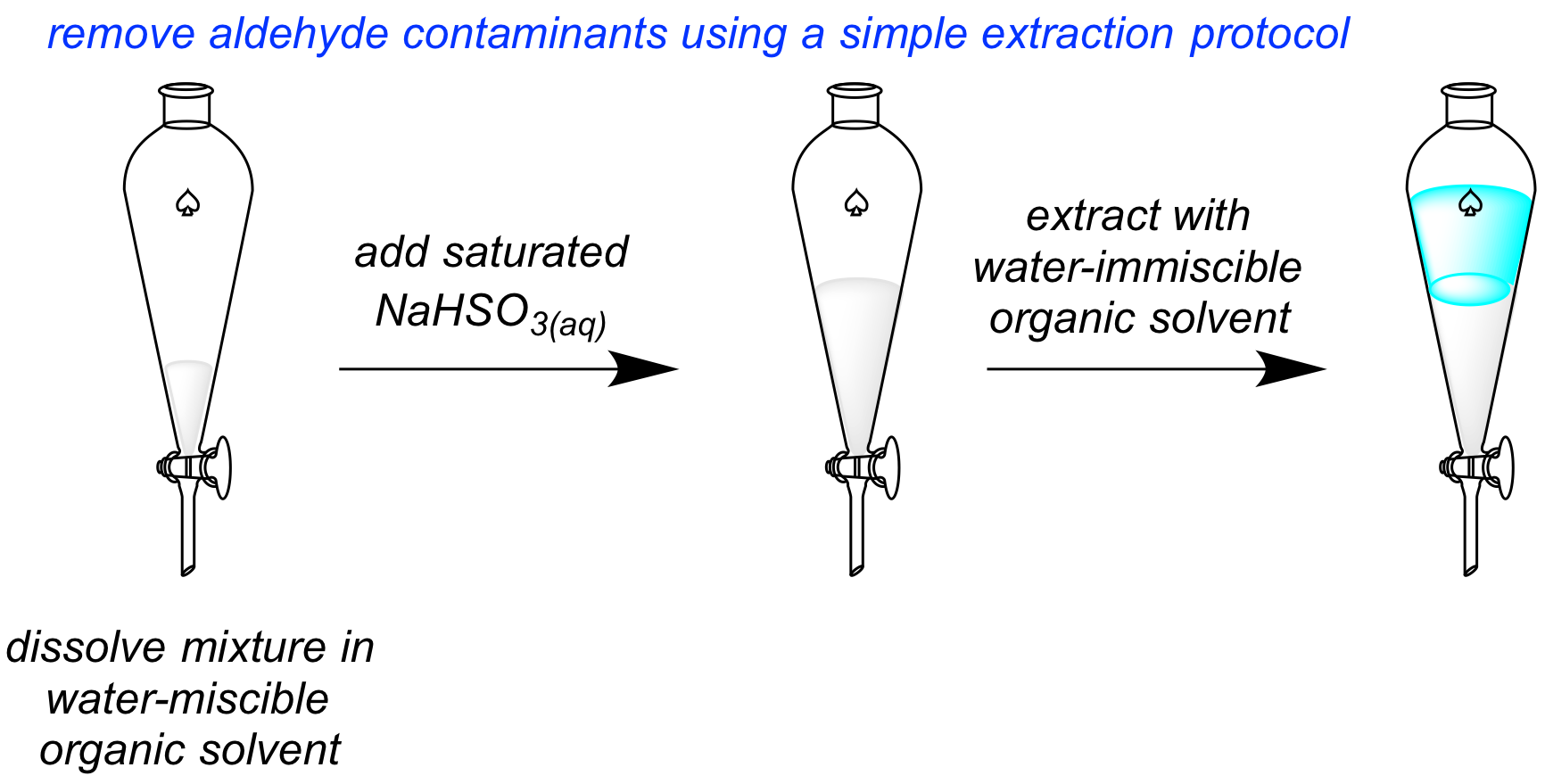
During the course of these studies, Phong Quach experienced difficulty in isolating one of our bisindolylmethane products in pure form. The material was contaminated by aldehyde starting material which co-eluted with the desired product. I encouraged him to investigate the use of bisulfite ion to selectively remove the aldehyde contaminant, by imparting a charge that would make it water-soluble during and aqueous extraction.
Our initial attempt was unsuccessful. We hypothesized that the amount of contact between the layers was insufficient, so I encouraged Phong to investigate using a miscible solvent first, to establish better contact with bisulfite ion, and then introduce an immiscible solvent to extract away the uncharged desired product from the aqueous-soluble bisulfite adduct. This strategy worked amazingly well. Given the ubiquity of aldehydes in organic synthesis, this method should prove widely applicable to the field of organic chemistry. I therefore explored optimization of the method, the substrate scope, functional group tolerance, and scale-up, together with undergraduates Maxwell Furigay and Maria Boucher. We completed this work over the summer and published our findings in Organic Process Research & Development. Gratifyingly, within three days of our work's public availability in the "Just Accepted" category of the website, we received an email from a researcher who had already found the work useful to their research. I am going to give a talk on this project at the Fall ACS meeting on Aug 22nd, 2017.
In summary, we have completed the double addition bisindoylmethane project and are nearly finished with the single addition project. We also explored a side project inspired by difficulties encountered with this work resulting in a highly successful method to remove aldehyde contaminants using simple aqueous extraction. This work will have a major impact on the chemical community. In the future we plan on investigating new chemical reactions catalyzed by trityl cations, as well as exploring what other types of reactions might be facilitated by the use of miscible solvents as part of an extraction protocol.