Reports: ND556455-ND5: Rejuvenating Conjugated Polymer Membranes for Oily Water Treatment
Eui-Hyeok Yang, PhD, Stevens Institute of Technology
In this work, we investigated how a nanostructured PPy(DBS) surface enhances its tunable wettability and droplet mobility compared to flat (non-structured) surfaces, by examining the changes of the apparent contact angles and the sliding angles of organic droplets during the redox processes.
We synthesized PPy(DBS) nanostructures using a maskless one-step oxygen plasma etching technique as shown in Figure 1. Frist, the electropolymerization of PPy(DBS) on the Cr/Au-coated silicon was done by applying a voltage of 0.8 V (vs. SCE) using potentiostat until the accumulated charge density reached 1000 mC.cm-2, corresponding to the PPy(DBS) film thickness of 4.7 μm. Then, the prepared PPy(DBS) sample was etched using an inductively coupled plasma under the fixed O2 flow rate of 30 sccm and chamber pressure of 20 Pa, different etching powers and times for nanopatterning the PPy(DBS) surface.
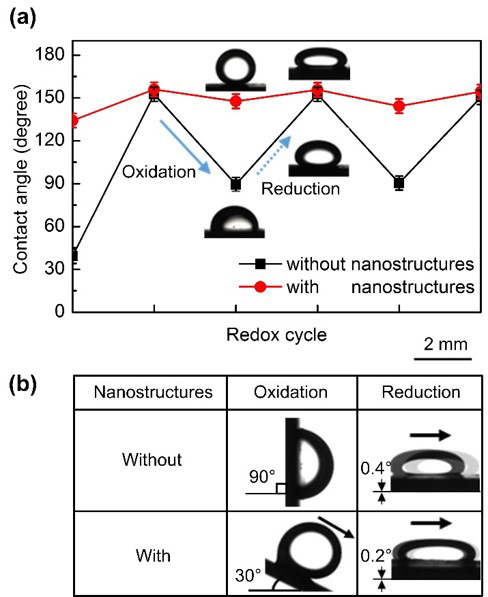
To investigate the effects of nanostructures on the tunable wettability and mobility of organic droplets on the PPy(DBS) surfaces under the immersion within electrolyte, the apparent contact angles and the sliding angles of DCM droplets on the PPy(DBS) surface with the nanostructures were examined. On the oxidized PPy(DBS) surface without nanostructures, the DCM droplet showed a contact angle of 89 ± 5° (Figure 1a) and was pinned on the surface (Figure 1b). Under reduction, the contact angle of the DCM droplet increased to 153 ± 3° along with the flattening of the droplet. The droplet rolled off from the reduced surface at the sliding angle of 0.4° and the contact angle hysteresis was 9 ± 4°, showing a dramatic change in the droplet mobility via the redox process. As for the DCM droplet on the PPy(DBS) surfaces with nanostructures, the initial contact angle under oxidation (148 ± 5°) was much larger than that without nanostructures (89 ± 5°). The dramatic increase of the contact angle even under the same oxidation state is due to the high-aspect-ratio pillared nanostructures, which support the droplet being in the Cassie-Baxter wetting state. In addition, the discrete pillared nanostructures reduce the effective contact line density and the pinning so that the contact angle hysteresis and the retention force between the droplet and the surface are greatly decreased. As a result, the DCM droplet on the oxidized surface featured with nanostructures also rolled off at the moderate sliding angle of 30 ± 3°, whereas that on the oxidized surface with no nanostructures was completely pinned even at the vertically titled angle of 90° (Figure 1b). The contact angle hysteresis on the oxidized surface with nanostructures was 38 ± 10°, which is also significantly lower than that without nanostructures. Such a low sliding angle and a contact angle hysteresis further indicate that the DCM droplet sits on the nanostructured PPy(DBS) surface in the Cassie-Baxter wetting state.
The nanostructured PPy(DBS) surface also allowed the change of its wettability and droplet mobility during reduction. While the contact angle of the reduced PPy(DBS) surface with nanostructures (158 ± 3°) was higher than that on the surface without nanostructures (153 ± 3°), the reduction of the oxidized surface with nanostructures increased the droplet contact angle no more than by ~10° (from 148° to 158°), which was much lower than that on the PPy(DBS) surface without nanostructures (~64°, from 89° to 153°) (Table 1). It should also be noted that while the contact angle hysteresis (4 ± 2°) and sliding angle (0.2 ± 0.1°) of the DCM droplet on the reduced PPy(DBS) surface with nanostructures are lower than those on the reduced surface with no nanostructures (9 ± 4° and 0.4 ± 0.1°, respectively), the differences are not as dramatic as in the case of the oxidized surface. It indicates that the impacts of the nanostructures of the PPy(DBS) surface on the oleophobicity and the droplet mobility are more significant in the oxidized state than the reduced one. It is because the release of DBS- molecules during the reduction, which decreases the droplet-electrolyte interfacial tension, is the more dominant effect on the contact angle increase and the droplet mobility than the change of the wetting state and the contact line density by the nanostructures.
In summary, we have investigated PPy(DBS) surfaces decorated with the high-aspect-ratio nanostructures for tunable wettability and droplet mobility. The droplet manipulation under redox processes was facilitated by the high-aspect-ratio nanostructures on the PPy(DBS) surfaces, compared to the flat surface with no nanostructures, because the solid-droplet contact line density was significantly reduced by the nanostructures supporting the Cassie-Baxter wetting state.
This grant provides support the PIs to pursue the study of the oily water treatment and the regeneration of membranes (self-cleaning). The support by ACS PRF enables us to work on elucidating the switchable adhesion of oils on polymeric membranes and utilizing these properties for oily water treatment and regeneration of membrane materials. This a very important support. Furthermore, with the funding provided by ACS PRF, one PhD student was supported to study the state-of-art control of droplet mobility on conjugated materials, which expanded the student’s research area and made the student more competitive in his career.
Table 1. Apparent contact angle, sliding angle, advancing/receding angle, and contact angle hysteresis (CAH) of a DCM droplet on PPy(DBS) surfaces without and with nanostructures at different redox states.
Nano-structures |
Oxidized |
Reduced |
||||||
θi (°) |
θs (°) |
θa/θr (°) |
CAH (°) |
θi (°) |
θs (°) |
θa/θr (°) |
CAH (°) |
|
Without |
89 ± 5 |
Pinned |
Pinned |
Pinned |
153 ± 3 |
0.4 ± 0.1 |
160/151 ± 2 |
9 ± 4 |
With |
148 ± 5 |
30 ± 3 |
161/123 ± 5 |
38 ± 10 |
158 ± 3 |
0.2 ± 0.1 |
161/157 ± 2 |
4 ± 2 |
Figure 1. Tunable wettability and mobility of the DCM droplet on the PPy(DBS) surfaces featured with nanostructures under the immersion within electrolyte, compared to those with no nanostructures. (a) The changes of apparent contact angles with respect to redox cycle. (b) Droplet mobility (sliding behaviors) on oxidized and reduced surfaces.