Reports: UR954875-UR9: Tailoring Ionic Liquids for Deep Desulfurization of Liquid Fuels by Oxidative Extraction
Hua Zhao, PhD, University of Northern Colorado
During the second year of the ACS-PRF funded project, the following major tasks have been successfully accomplished:
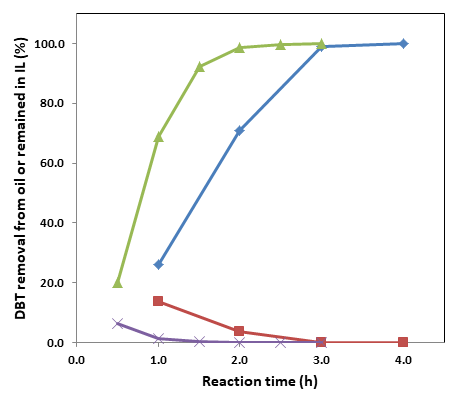
1. Following the successful identification of ionic liquids for efficient extraction of aromatic sulfur compounds from fuels in Year 1, we further continued our study on the use of these ionic liquids for oxidative extraction removal of sulfur compounds. The deep desulfurization of petroleum-based fuels using oxidative extraction processes has been hampered by the lack of suitable solvent systems and by the poor availability of inexpensive and efficient catalysts. As a strategy to address this limitation, we evaluated a number of hydrophilic and hydrophobic ionic liquids (ILs) for the oxidative extraction of dibenzothiophene (DBT) and other aromatic sulfur compounds (e.g., thiophene, TS, benzothiophene, BT, and 4,6-dimethyldibenzothiophene, DMDBT) from n-octane using H2O2 as the oxidant and V2O5 as the catalyst. Our findings are significant for petroleum desulfurization and suggest that ILs for effective sulfur removal will typically comprise low basicity/nucleophilicity anions paired to cations possessing some hydrophilic character. Among a number of suitable ILs identified for this application, [Choline][Tf2N] is particularly promising as a relatively low-cost solvent ideal for use in oxidative extraction to desulfurize fuel. The [Choline][Tf2N]‒V2O5 system afforded essentially quantitative removal of DBT, BT, and DMDBT at room temperature, even for initial sulfur levels as high as 3,000 ppm (Figure 1). This advance enables a highly efficient and cost-effective desulfurization process that has a promising industrial application for producing ultra-low-sulfur fuel.
Figure 1 Time course of DBT removal from n-octane by oxidative extraction: diamond symbols for DBT removal (%) from oil with 500 ppm S; square symbols for % DBT remaining in IL contacted with oil having initial S of 500 ppm; triangle symbols for DBT removal (%) from oil with 3,000 ppm S; and checkmark symbols for % DBT remaining in IL contacted with oil having initial S of 3,000 ppm (Conditions: 1.0 mL of IL, 1.0 mL of n-octane (oil) containing 500 or 3000 ppm S derived from DBT, 40 µL of 30% H2O2, 1.0 mg of V2O5, conducted with gentle stirring at room temperature, ~22 ºC).
2. Two undergraduate students were supported by this project to participate in the research activities. Both students received safety training and hands-on training on instruments (including HPLC, NMR and Karl Fisher titrator). Both students assisted the PI in surveying the literatures, setting up the experiments, collecting and analyzing samples, and calculating data. The research experience made a huge impact on both students’ choices of career: one student graduated in May 2017 with a BS degree and is applying for dental school, and the other student is expected to graduate in a year with a BS degree and is planning to apply for graduate school.