Reports: ND1054806-ND10: Thermomechanical Reactions of Volatiles in Deep Earth Environments: Formation and Evolution of Abiotic Hydrocarbons
Choong-Shik Yoo, Washington State University
Thermomechanical Reactions of Volatiles in Deep Earth Environments: Baro- and Photo- Chemistries in Dense H2S and H2+S
Summary: This project is to investigate the formation and evolution of the Earth's volatiles at high pressure-temperature conditions deep in the Earth's interior. In this reporting period, we have investigated baro- and photo-chemical reduction processes in H2S and H2+S in diamond anvil cell, using in-situ Raman spectroscopy. The results show spectral evidences for the pressure-induced polymerization of H2S to 'polymeric' phase V at 30 GPa at 300 K, analogous to an extended network structure of ice-X with symmetrized hydrogen bonds. This process, however, competes with strong photochemical activities in dense H2S, as also found in H2+S, which presumably leads to H3S – the record high Tc superconductor with the critical temperature at 204 K at 200 GPa.
Research Objectives: Hydrogen sulfide is a volcanic gas with a characteristic odor of rotten eggs. It also exists in natural halite rock salts, which makes it important to understanding the chemistry of Earth's early atmosphere and prebiotic synthesis. It is also found in nearly all types of atmospheres on terrestrial exoplanets. In addition, H2S is an important chemical compound produced in deep, mid-ridge ocean hydrothermal vents via bacterial breakdown of organic materials in anaerobic conditions, as well as in the human body, which uses it as a signaling molecule for a variety of physiological effects.
Solid H2S, like its chemical analog H2O, is a typical hydrogen-bonded molecular crystal; yet, unlike H2O, the stability and chemistry of dense H2S is substantially more complex and less understood. Therefore, the objective of this research is to investigate the pressure- and photo-induced transformations in H2S and S+H2 and understand the phase stability, hydrogen bond ordering, and chemical mechanism to H3S.
Progress in this reporting period:
A. Phase diagram of H2S
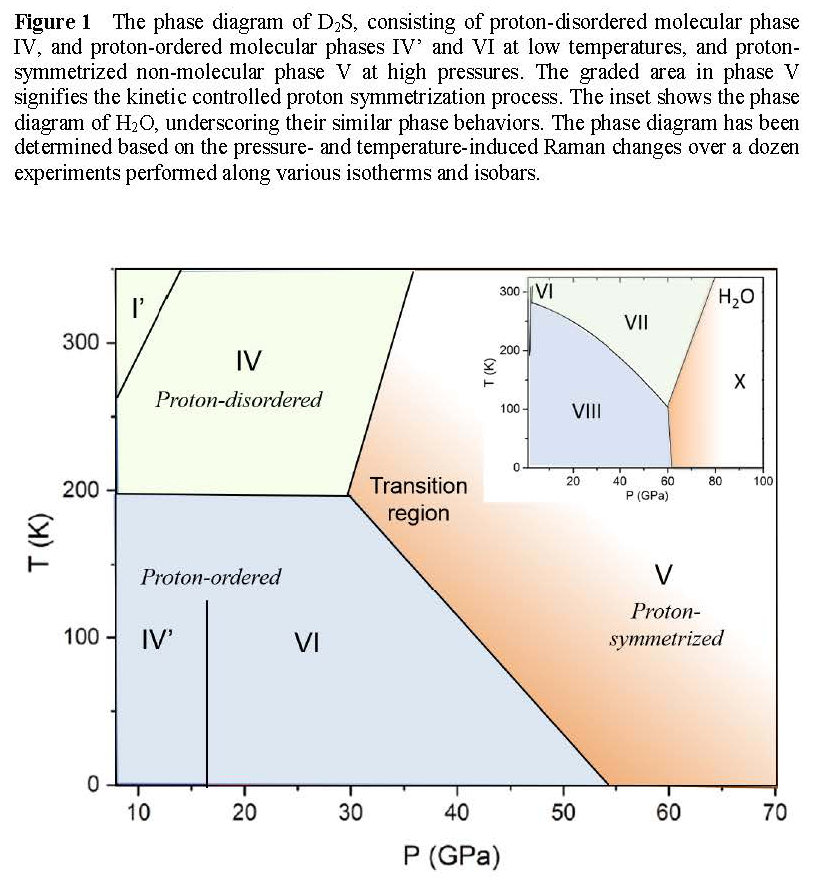
Solid H2S, like its chemical analog H2O ices, is a typical hydrogen-bonded molecular crystal; yet, unlike H2O, the stability and chemistry of dense H2S is substantially more complex and less understood. The accurate determination of phase diagram of dense H2S is, therefore, critical to understand the phase stability, hydrogen bonding, and chemistry of H2S. Therefore, we have determined the phase diagram of D2S to 70 GPa in diamond anvil cells using confocal micro-Raman spectroscopy (Figure 1). The results show molecular phase IV transforms to 'polymeric' phase V at 30 GPa at 300 K, analogous to an extended network structure of ice-X with symmetrized hydrogen bonds. The formation of symmetrized phase V is evident by its characteristic single vibrational Raman peak at ~460 cm-1 for symmetric D-S-D bending/stretching, analogous to that of ice X at ~730 cm-1 at 76 GPa (Figure 2). At low temperatures, the proton-disordered phase IV transforms to more proton-ordered phases of IV' and VI, which also transforms to phase V at 40 GPa at 100 K. The present phase diagram indicates that D2S is chemically stable at least to 70 GPa at 300 K, contrary to the previously reported decomposition to sulfur and HxS (x>2) above 30 GPa. Nevertheless, we found strong photochemical activities in dense H2S, which competes with the observed barochemical process. This conclusion is important, considering that a wide range of decomposed states (H3S + S, H3S+ + HS-, etc.) was used to explain recently observed high Tc superconductivity in dense H2S.
B. Photochemical Reactions in Sulfur + H2 Mixture
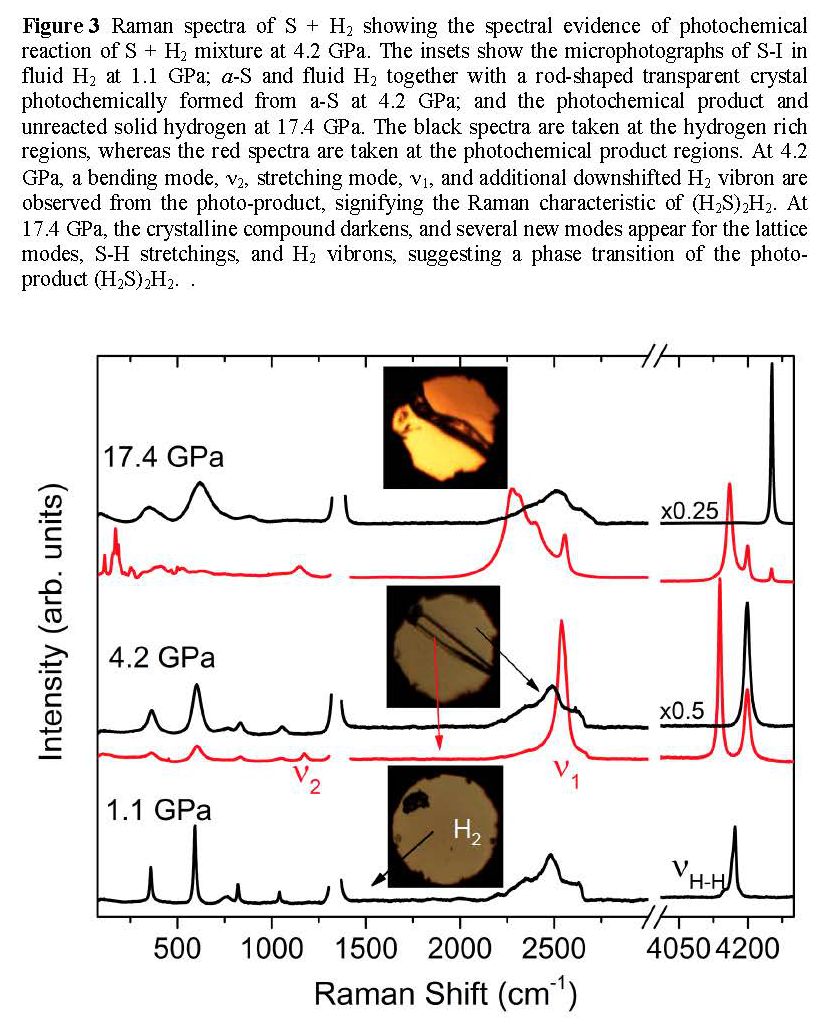
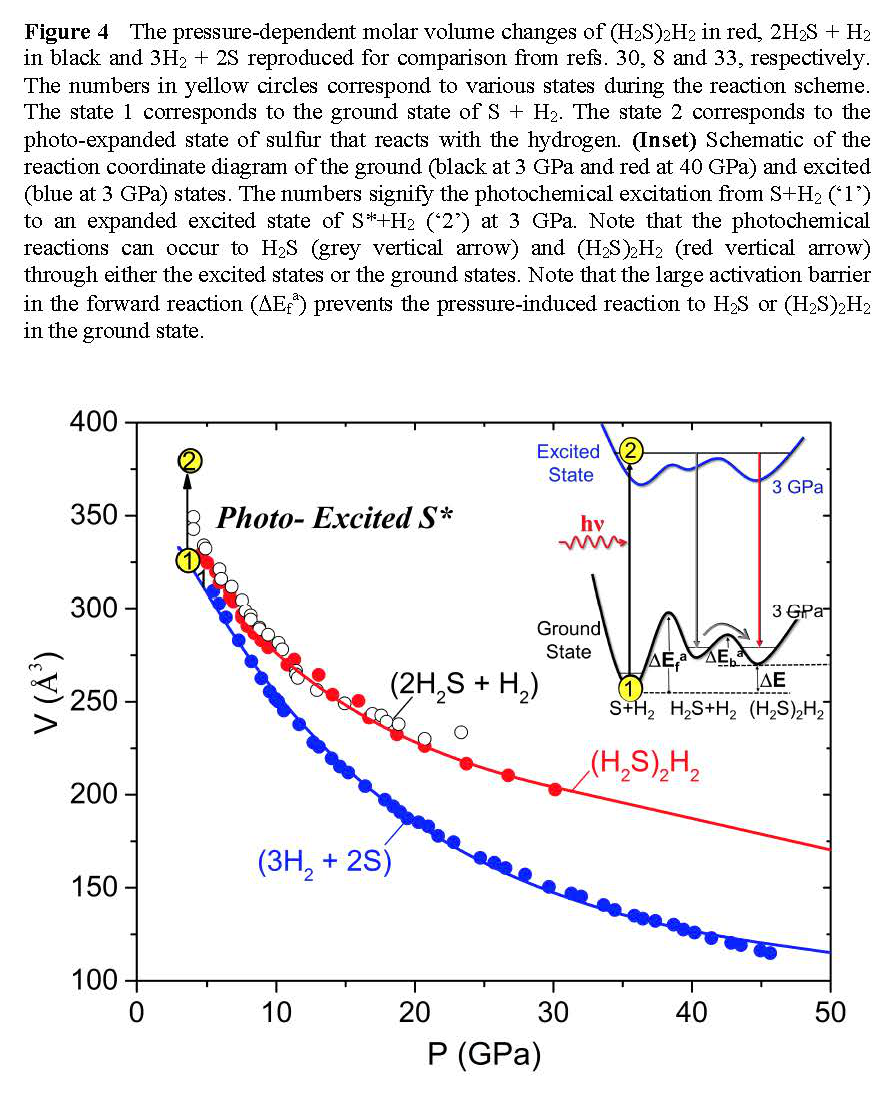
Photochemical effect is important for understanding high-pressure behaviors of sulfur and sulfur-containing materials including H2S and H3S. Therefore, we have investigated the pressure- and photo-induced transformations of S+H2 mixtures to 50 GPa. The results show that the photochemical transformation occurs in the mixture to (H2S)2H2 through an expanded state of photoactive a-S phase of sulfur at 3 GPa. Upon further compression, the photoproduct (H2S)2H2 undergoes a structural phase transition at 17 GPa and then decomposes back to sulfur (S-III) and hydrogen above 40 GPa. The pressure-induced Raman changes indicate that the phase transition at 17 GPa is associated with the proton-ordering process in H2S, evident by the profound splitting of S-H and H-H vibrational modes (Figure 3). The pressure-induced decomposition, on the other hand, is driven primarily by densification, which occurs at 40 GPa where the molar volume of (H2S)2H2 becomes substantially larger (by 25%) than that of S + H2 mixtures (Figure 4). Without influence of lights, no pressure-induced chemical change was observed in the S+H2 mixture to 50 GPa, underscoring the significance of the photochemical effect in this mixture.
Significance of the Result: High-pressure chemistry is a thermal process that occurs through compressed states, either the ground states at given densities or near ground states that are kinetically constrained by large activation barriers. In contrast, the photochemical reaction is an athermal process that occurs through an expanded excited state. Therefore, the reaction pathways of the two processes are greatly different, especially at low pressures (< a few GPa), because of relatively large energy differences (a few eV) between the ground and excited electronic states (i.e., the band gap) with respect to relatively small compression energy (typically less than a few tenth of eV). As such, the former results in denser products, whereas the latter results in expanded products for bound electronic potentials or decomposition products for unbound excited states. Nevertheless, the two processes become relevant at high pressures (> 10-30 GPa), where the compression energy (i.e. DE = PDV) becomes comparable to the band gap energy. Such a path-dependent transformation is also important to understand the chemical mechanism to H3S and can be used to control high-pressure chemistry and synthesize novel materials.
Figure 1 The phase diagram of D2S.
Figure 2 Pressure-induced Raman changes of D2S along the 300K-isotherm.
Figure 3 Raman spectra of S + H2 showing the spectral evidence of photochemical reaction of S + H2 mixture at 4.2 GPa.
Figure 4 The pressure-dependent molar volume changes of (H2S)2H2 in red, 2H2S + H2 in black and 3H2 + 2S.