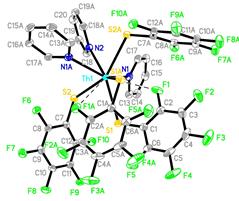
Reports: ND352382-ND3: Nanoscale Actinide Clusters
John Brennan, PhD, Rutgers, the State University of New Jersey (New Brunswick)
Under conditions that
lead to formation of chalcogenide phases (reaction 1), the fluorinated
compounds both decompose to give ThF4 (reaction 2), with no evidence
for the formation of either chalcogenide or oxide phases. This reactivity is
characteristic of the more electropositive metals. A comprehensive analysis of
the volatile products is still in progress.