Reports: ND351971-ND3: Metal-Free Activation of Nitrogen Oxides for Hydrocarbon Functionalization
Timothy H. Warren, Georgetown University
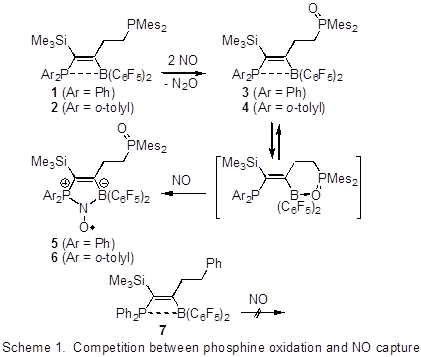
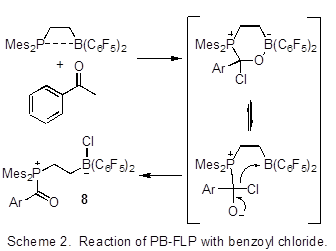
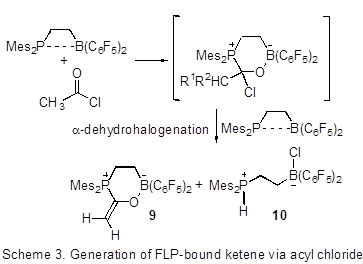
Timothy H. Warren, Georgetown University

Copyright © American Chemical Society