Reports: DNI552636-DNI5: Effects of Oxygen Vacancies of Catalyst Surfaces on Reactivity and Selectivity of Carbon Dioxide Reduction with Hydrogen
Franklin (Feng) Tao, PhD, University of Notre Dame
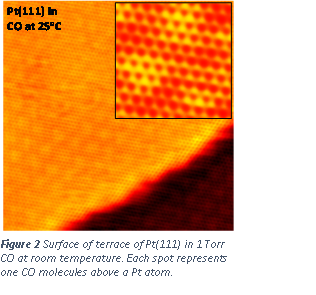
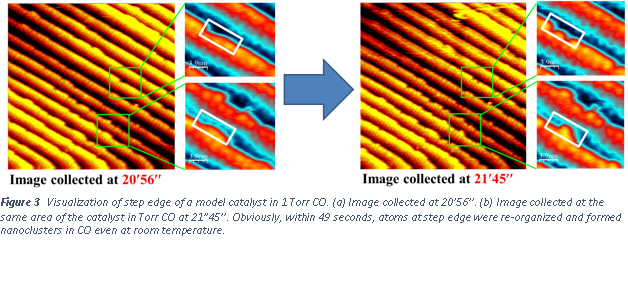
Through the support from the ACS-PRF Doctoral New Investigator grant in the period of 2012-2014, we have performed fundamental understanding of surface of model catalysts through a new instrument developed in our group, high pressure high temperature STM (Figure 1). Student, Luan Nguyen and Shiran Zhang have worked on this project. We have successfully imaged the surface of a platinum model catalyst with a flat (111) surface. Atom-resolved STM images on the terrace of Pt(111) in the gas environment of CO with a pressure of 1 Torr or higher were achieved (Figure 2). Here CO is a probing reactant molecule. The goal is to elucidate how pressure of a reactant gas could change surface structure, particularly the step edge in terms of atomic packing at step edge. We tracked the packing of atom at step edge as a function time. We have first revealed the formation of Pt nanoclusters at the edge of Pt surface driven by the existence of reactant gas. Figure 3 clearly shows the time-dependent restructuring at a step edge and formation of nanoclusters within one minute. In UHV, there is no such a restructuring observed.
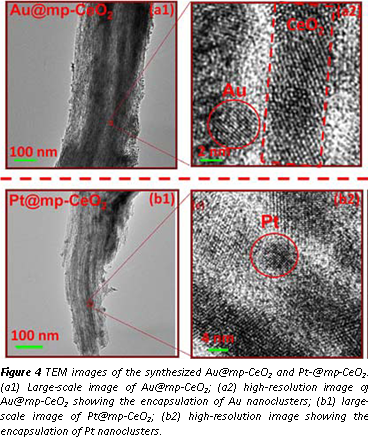
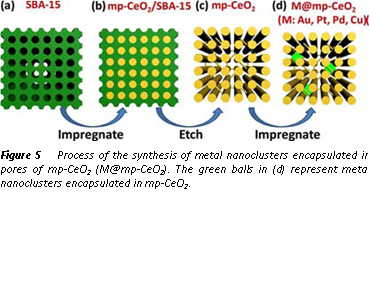
With the support of ACS-PRF ACS-PRF Doctoral New Investigator grant, we have also developed low temperature catalysts which converts CO with H2O to H2 and CO2. This work is driven by the development of efficient low temperature catalysts for removing CO in H2 source feeding to low-temperature hydrogen fuel cells. Different from the metal nanoparticles supported on oxide particles, we prepared new catalysts which are metal nanoclusters (<5 nm) supported on the internal surface of meso-pores of mp-CeO2. Figure 4 shows the catalysts synthesized with two steps. The first step is the synthesis of a mesoporous CeO2 with through a hard template of mesoporous SiO2. In the second step, metal nanoparticles are formed on the internal surface of the pores of mp-CeO2 through deposition precipitation with a following reduction in H2. Figure 5 schematically presents the method of preparation of new catalyst metal nanoclusters capsulated in mp-CeO2. We performed kinetics studies for water-gas shift on this catalyst and the counterpart, metal nanoparticles supported on CeO2 nanorods. We found that the new catalysts, metal nanoparticles capsulated in mp-CeO2 exhibit low activation barriers for WGS than metal nanoparticles supported on CeO2. Theselower activation barriers suggest the bent internal surfaces of metal and oxide plays a different role in terms of catalyzing water-gas shift. It further suggests that the formation of a bent interface could be a method to develop catalysts with high activity. We achieved fundamental understanding of this difference between the two series of catalyst through in-situ studies using our ambient pressure XPS. Oxygen vacancies on a CeO2 surface of the two types of catalysts were measured. We found the bent internal surface exhibits larger density of oxygen vacancies. With the correlation between surface chemistry of the catalysts and the corresponding catalytic performances, we conclude that the surface chemistry of the bent metal-oxide interface plays a different role compared to the interface between metal nanoparticles supported on a flat CeO2 nanoparticles.
With the same synthesis, we prepared other three catalysts Pd NPs@mp-CeO2 and Au NPs@mp-CeO2, and Cu NPs@mp-CeO2; our catalysis studies show they exhibit higher activity than the metal nanoparticles supported on external surface CeO2 nanorods. Overall, this work suggests a new method for development of new WGS catalysts with high activity and low activation barrier.
Other than two projects, we have worked on reduction of CO2 with H2 and other reducing gases. We have prepared CeO2-based catalyst with anchored Ni cations and Ru cations. This catalyst exhibits high activity for the reduction of CO2. We are preparing a manuscript of this work.