Reports: DNI1053668-DNI10: Synthesis of Nanostructured Multi-Metallic Carbide Materials for Inexpensive Catalysts and Catalyst Supports
Brian Leonard, PhD, University of Wyoming
1. Synthesis of metal carbide nanomaterials
Metal carbide compounds have numerous
opportunities as economical catalysts and catalyst supports. In order to
investigate their catalytic properties, the synthesis of these materials as
high surface area nanomaterials needs to be explored. We are developing a novel
synthesis technique that allows us to produce carbide nanomaterials with shape
and composition control. Utilizing a combination of simple salts, a molten flux
can be created which aids in diffusion of the solid state reactants and
dramatically lowers the reaction temperatures and times allowing access to
metal carbide nanomaterials. The reactants chosen for these materials are fine
metal powders (ex. Ta, Mo, W etc.) and multi-wall carbon nanotubes (MWCNTs).
The MWCNTs provide carbon for the final metal carbide product and also serve as
a template on which the metals deposit and react. The final products are metal
carbide nanomaterials with wire-like shapes and sizes similar to the original
MWCNTs. As seen in Figure 1, a variety of phase pure metal carbide compounds
can be synthesized using this technique. 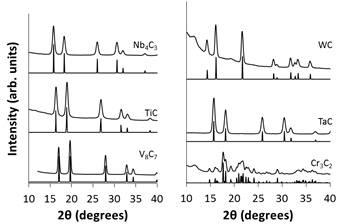
Figure 1. X-ray Diffraction patterns showing phase pure single metal carbide products. Below each experimental diffraction pattern is the calculated data from the PDF4+ database
X-ray diffraction of the products confirms they are pure materials void of any oxide or metal impurities. To confirm the morphology of these materials, Transmission electron microscopy was used to image the carbide materials. Figure 2 shows selected examples of the carbide nanomaterials.
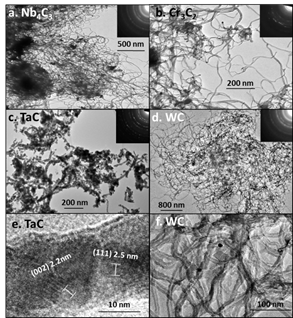
Figure 2. TEM images of metal carbide nanomaterials. Inset images show SAED of the material confirming the nanomaterials are metal carbides.
The TEM images highlight the nanowire morphology of these carbide materials. The diameter of the wires has increased with the incorporation of the metal forming the metal carbide compounds. MWCNTs as received have a diameter of 8-10 nm while the carbides have diameters between 15-30nm. In addition, the carbide nanowires typically have a solid interior compared to the hollow carbon nanotubes. While the size of the wires has increased, the final products still have narrow widths and thus higher surface areas that traditionally synthesized metal carbide materials. Using BET surface area measurements, we have found that our carbide materials have surface areas between 10 and 50m2/g.
2. Bimetallic carbides
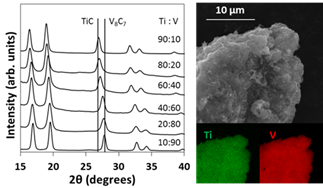
Bimetallic carbide compounds allow much greater opportunities for fine tuning catalyst activity through lattice spacing, electronic structure, and crystal structure tuning. By incorporating two metals into the synthesis procedure, we have been able to produce a range of bimetallic carbide compounds. The first example is Ti-V-C. Both TiC and V8C7 form in the rock salt crystal structure. This allows for a solid solution of TiC-V8C7 with complete homogeneity. By changing the amount of starting metals, we have synthesized and characterized a range of Ti-V-C materials with controllable composition. In Figure 3, the XRD patterns of the materials shifts as the composition changes. This is due to the different sizes of metals and thus different lattice constants.
Figure 3. Left, XRD of TiVC nanomaterials with tunable composition. Right, SEM with EDS mapping of TiVC sample showing the presence of Ti and V in all regions confirming the homogeneous bimetallic nature of the product.
In addition to lattice constant changes, Ti-V-C was also investigated by Energy Dispersive Spectroscopy (EDS) mapping. This technique gives the elemental composition of the sample and revealed a homogeneous composition throughout the sample within 5% of the starting composition further confirming the XRD results. This kind of composition control has never been seen in the nano size regime and is further evidence that this synthesis technique’s power.
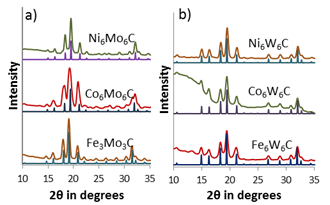
The synthesis of bimetallic carbides was further extended to include late transition elements like Ni, Fe, and Co. Carbides of these metals are frequently less stable and form different structures than their early transition metal counterparts. To increase the stability of these materials, they were combined with Mo and W which are known to form very stable materials. The results were carbides with the general formula of M6(Mo/W)6C where M is Fe, Ni, and Co. Figure 4 shows the XRD patterns for these late transition metal products.
Figure 4. Bimetallic carbide compounds of Fe, Co, Ni, combined with Mo and W.
These carbides have a unique crystal structure and variable carbon content which will be further investigated for effects on catalytic activity. In addition, several of these bimetallic systems for variable composition phases like M2(Mo/W)4C and M4(Mo/W)2C which will be synthesized and evaluated for their catalytic activity. We are also investigating multiple bimetallic systems of the early transition metals specifically Ta-W-C and Ta-Mo-C. We believe that these materials have will have a similar electronic structure to precious metal catalysts.
3. Catalyst supports
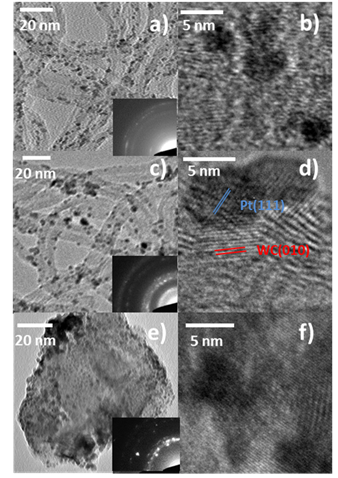
We are also investigating these metal carbide materials as catalysts supports. By replacing the traditional carbon black support material with a more robust metal carbide material, we can greatly extend the lifetime of precious metal catalysts. Metal carbide compounds have some of the highest melting points of all compounds and are quite resistant to oxidation and chemical attach making them ideal candidates for use as support materials. In addition, support catalyst interactions often affect the activity of the catalyst through synergistic effects. To investigate these effects, we have begun depositing Pt nanoparticles on metal carbide nanowires. Using a wet impregnation technique, we deposited Pt nanoparticles of 3-5 nm with good dispersion and size control. Figure 5 shows some sample TEM images of the metal carbide supported Pt nanoparticles. These materials will be investigated in the coming months for their catalytic activity and support interactions.
Figure 5. TEM images of Pt/Mo2C (a and b), Pt/WC (c and d) and Pt/TiC (e and f). Insets show the SAED patterns of for each sample confirming the presence of carbide and Pt.