Reports: UNI152945-UNI1: Development of Copper(I) Catalysts for Photoredox Catalysis
Katrina H. Jensen, PhD, Black Hills State University
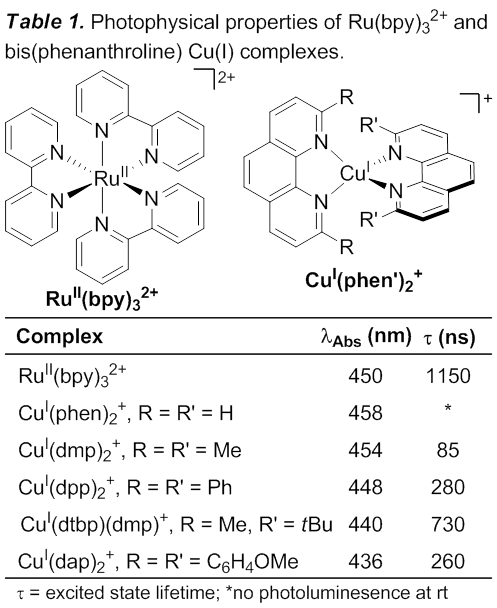
The goal of this project is to develop copper(I) catalysts for photoredox reactions, where visible light is used to facilitate redox reactions. In these reactions, the photoredox catalyst absorbs a photon and then either donates an electron to or accepts an electron from an organic molecule to generate a reactive radical intermediate which has significant synthetic potential. Light can be considered a reagent in such reactions, which is both economical and environmentally friendly. The pioneering work in this field involves the use of ruthenium and iridium photocatalysts;1 however these metals are both expensive and toxic. We are currently investigating catalysts that use copper, which is an earth abundant element, rather than ruthenium or iridium, both of which are among the rarest metals. Bis(phenanthroline) copper(I) complexes are known with photophysical properties similar to proven photoredox catalyst Ru(bpy)3Cl2 (Table 1),2 and we hypothesized that they could act as effective catalysts for photoredox reactions.
To begin our
investigation, we chose to evaluate copper complexes in the context of a model
reaction which is known to proceed via photoredox catalysis. The
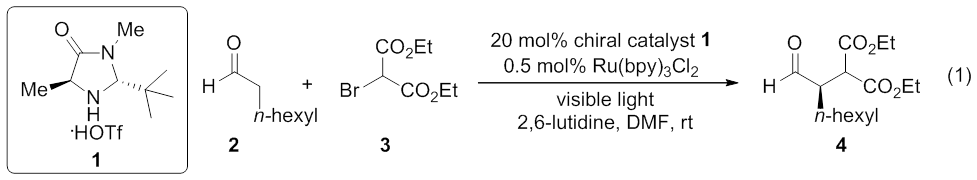
α-alkylation of aldehydes catalyzed by Ru(bpy)3Cl2 as
a photoredox catalyst and imidazolidinone 1 as a chiral catalyst was
selected as a reaction for evaluation (equation 1).3 We began our
analysis using similar reaction conditions, but replacing the photoredox
catalyst Ru(bpy)3Cl2 with bis(phenanthroline) copper(I)
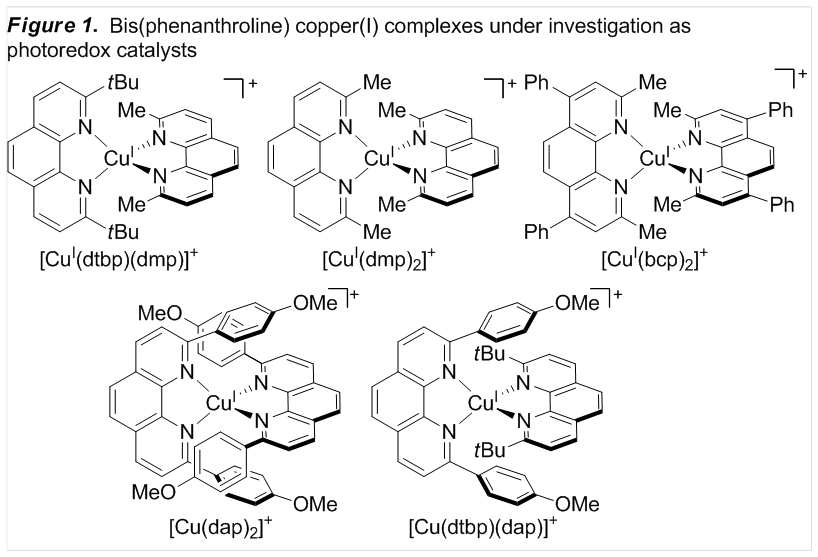
complexes (Figure 1). Based on the long excited state lifetime of
Cu(dtbp)(dmp)+ (dtbp = 2,9-di-tert-butyl-1,10-phenanthroline,
dmp = 2,9-dimethyl-1,10-phenanthroline), we expected this complex to
potentially be the most promising catalyst. Under these conditions, however,
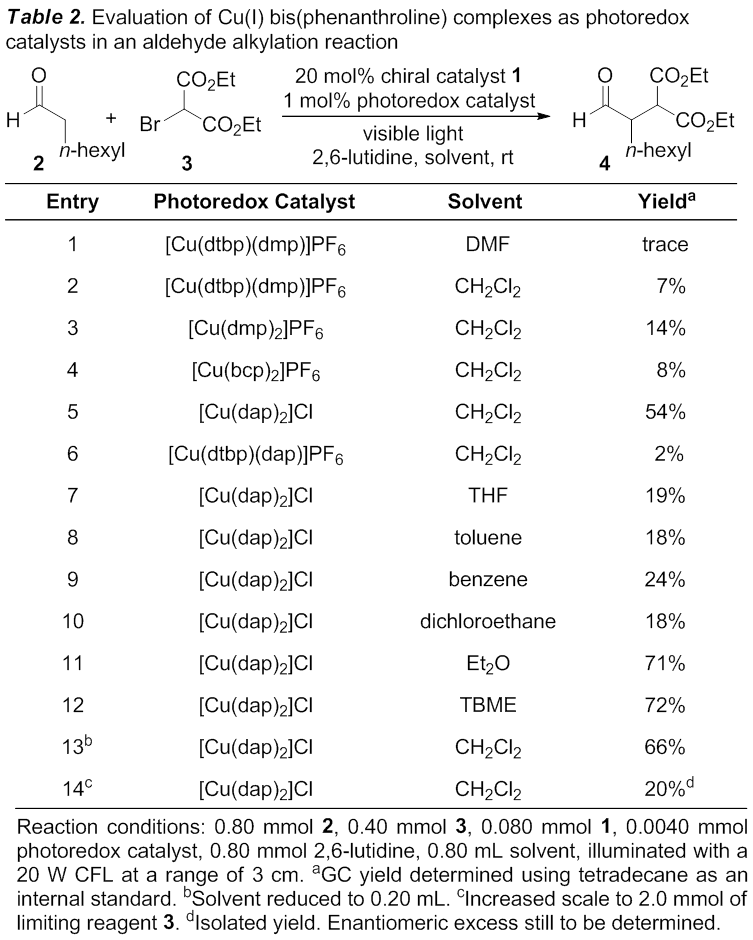
only a trace amount of the desired product 4 was observed by GC-MS
(Table 2, entry 1). After isolating authentic product 4, we developed an assay using gas chromatography with an
internal standard to quantify reactant conversion and product formation without
the need for chromatographic product isolation. Due to the
known impact that solvent can have on the excited state lifetime of copper
complexes, we chose to switch to a noncoordinating solvent, dichloromethane,
for the evaluation of other copper catalysts. Cu(dmp)2PF6,
Cu(bcp)2PF6, Cu(dap)2Cl, and Cu(dtbp)(dap)PF6
(bcp = bathocuproine, dap = 2,9-di-para-anisole-1,10-phenanthroline) were
all synthesized and evaluated as catalysts (entries 2-6), with Cu(dap)2Cl
giving the highest yield. We next evaluated a variety of solvents, and found
that ethereal solvents (diethyl ether and tert-butyl methyl ether)
slightly increased product yield (entries 11 & 12). We also observed that
increasing the reaction concentration improved product yield (entry 13). Unfortunately
the product yield decreased upon a five-fold increase in the scale of the
reaction (entry 14). We are currently working to resolve this issue by taking
steps to better eliminate air from the reactions and testing alternative
glassware (considering the average depth that light must travel through the
reaction mixture). In the second year of this project, we will continue to
adjust the reaction conditions to improve product yield, determine the
enantiomeric excess of the product and then evaluate the scope of the reaction. During this
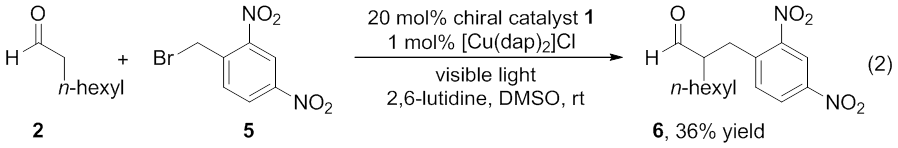
first year of the project we also explored another reaction, the photoredox
catalyzed α‑benzylation of aldehydes using electron poor benzyl
bromides (equation 2).4 Product 6 was isolated in 36% yield
with Cu(dap)2Cl as photoredox catalyst and DMSO as solvent.
Attempts to develop a GC assay for this reaction were unsuccessful, as the
product did not elute under any conditions we tried. Thus we developed an NMR
assay using dimethyl terephthalate as an internal standard and will use this
assay to facilitate reaction optimization. In the second year of the project,
we also plan to evaluate the scope of this reaction and explore new reactions
that may be catalyzed by copper(I) photoredox catalysts.
This project
provided research opportunities for two undergraduate students during the
academic year (one with PRF support, the other with a fellowship from the South
Dakota Biomedical Research Infrastructure Network) and five undergraduate
students during the summer (all with partial PRF support). Four poster
presentations were given by undergraduate students at local, regional, and
national conferences, including at the Black Hills Research Symposium, at the
annual meeting of the South Dakota Biomedical Research Infrastructure Network,
and at the National Conference on Undergraduate Research. Support from the Petroleum
Research Fund has allowed the PI to diversify her research program as she
establishes an independent research career.