Reports: UR451689-UR4: Exploring the Controllable Uptake and Release of Pyridinium Ions with a Photoactive Calixarene-Capped Azobenzene
Paul A. Bonvallet, PhD, College of Wooster
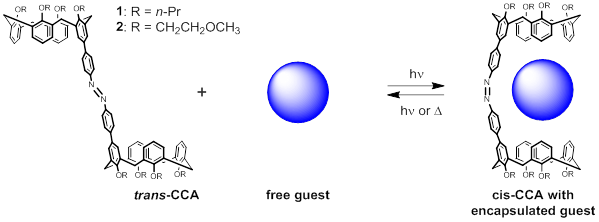
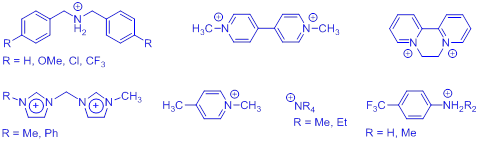
I. Technical progress. The overarching goal of the project is to develop a molecular capsule for the controllable uptake, storage, and release of cationic guests. By virtue of the convergent calix[4]arene hemispheres connected by a photoactive azobenzene bridge, we anticipate that this calixarene-capped azobenzene (CCA) host will be able to selectively entrap various pyridinium-, ammonium-, and diimidazolium-derived guest species shown below. The function of this light-activated capsule comes from the trans-cis photoisomerization reaction of azobenzene, whereby two geometrically distinct “open” and “closed” forms can be produced in response to light.
Guests: (counterions primarily I− or PF6−)
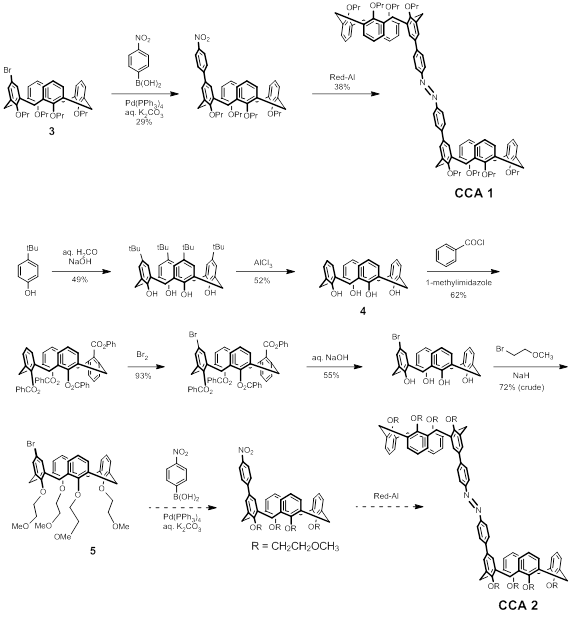
In the Year 1 report we described challenges in finding solvents for which both the host and the guest would be mutually soluble. CCA 1 is highly nonpolar (dissolves well in hexane, modestly well in chloroform) while the suite of cationic guests is almost universally insoluble in these solvents. After various ion exchange reactions in which the guests listed above were equipped with halide and/or hexafluorophosphate counterions, we concluded that we should focus instead upon the covalent modification of the pendant groups along the lower (narrow) rim of CCA in order to increase the solubility of the host. Thus we designed a new synthetic scheme for the derivative CCA 2 as shown in Scheme 1.
Scheme 1
The attachment of (poly)ethylene glycol chains to enhance solubility, known as “PEGylation,” is a well-known practice in medicinal chemistry and other fields. Thus a modified target CCA 2 that is equipped with 2-methoxyethoxy substituents (instead of n-propoxy) along the calixarene lower rim is expected to increase the solubility of the calixarene dimer in more polar organic solvents like acetone and acetonitrile.
The disadvantage of this strategy is the substantial extension of the synthetic route, both for the covalent attachment of the polyether group as well as the preparation of the necessary calix[4]arene precursor. Given that chemical vendors charge an average of $160 per gram for calix[4]arene (4), we decided to prepare this key precursor in our own laboratory due to the large amount of starting material that is necessary to carry out the multi-step synthesis. This decision added another two steps to the synthetic scheme but afforded us with many dozens of grams of 4 at a savings of more than $10,000 compared to the cost of purchasing from a vendor.
Our primary activities over the past year have thus been largely synthetic in nature, focusing on the eight-step scheme. We currently have a sizeable (more than one gram) supply of polyether 5 in a crude form in 6% overall yield, just two steps away from the target CCA 2. Preliminary indications are that this precursor 5 is much more soluble in chloroform than the analogous tetrapropoxy compound 3, which is a promising sign for the polyether being a solubility-enhancing group.
II. Impact on undergraduate development.
Virginia and Andrea both presented research posters at the 247th National Meeting of the American Chemical Society in Dallas, TX in March 2014. Andrea also presented at the day-long Senior Independent Study showcase held on campus every April.
The immediate benefit to this group of students is that they have the opportunity to focus on a research project in a way that is not possible during the busy academic year. While they are supervised closely, the students design their own experiments and execute them independently. They obtain their own data from instruments like our high-field NMR, and we had regular individual and small-group meetings for the communication of results. My students learned how to search the primary literature and adapt their procedures from research articles. They communicate their results both orally and in research posters and internal PowerPoint presentations.
Most importantly, these young scientists are able to engage in the scientific process firsthand, becoming creators of knowledge rather than simply consumers of knowledge.
III. Impact on PI development. The project supported by PRF has become one of the staples of my Senior Independent Study research with seniors at the College of Wooster. Over the past year I have presented the research at a national meeting of the ACS, both personally and as a co-author on two of my students’ ACS research posters. I will also be presenting this research at an invited seminar at Denison University next week.
Support for travel to the ACS meeting in Dallas was particularly useful during the past year – firstly because the College of Wooster does not cover all expenses, and secondly because of the opportunities for feedback and collaboration within a professional community. Following a presentation of this project at the ACS meeting I received some useful feedback about the project, a few ideas for the future, and new professional connections that may lead to a sabbatical research project. My laboratory’s expertise in lower-rim modification of calix[4]arenes was noticed by Professor Xiaopeng Li from Texas State University, who recently asked for some of our precursor molecules for a series of experiments that he is conducting on rigid metallo-supramolecular scaffolds. The ability to have students in my laboratory during the summer, coupled with the support for travel and dissemination of results, enables me to follow the teacher-scholar ideal that is valued at the College of Wooster.