Reports: DNI752019-DNI7: Bottom-Up Synthesis of Structurally Precise Graphene Nanoribbons
William Dichtel, PhD, Cornell University
We integrated the benzannulation reaction into a general strategy to produce larger aromatic systems by expanding its utility to silyl-protected acetylenes. Only a limited number of these substrates have been explored in benzannulation reactions and are unreported as substrates for the Cu(OTf)2 or ZnCl2-mediated reactions described below. Under typical benzannulation conditions, silyl groups are protodesilylated to provide the corresponding 2-arylnaphthalene. We modified the reaction conditions to retain the silyl group, such that they may be transformed to iodides and derivatized using cross-coupling reactions.
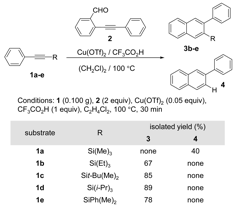
Table 1.
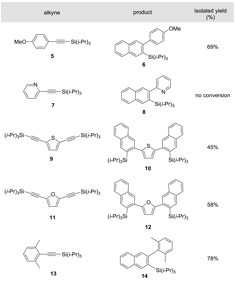
We first evaluated the benzannulation of phenyl acetylenes protected by silyl groups that differ in size and relative stability. Typical reaction conditions include excess CF3CO2H because its conjugate base is thought to promote the formation of naphthalenes over naphthyl ketone side products. These conditions provide desilylated 2‑phenylnaphthalene 4 as the major or only benzannulation product for various silyl-protected phenylacetylenes, even for relatively robust silyl groups. Reducing the initial concentration of CF3CO2H (1 equiv per alkyne) provides nearly complete retention of the silyl eithers in naphthalene products 3b-e (Table 1). Substrates 1b and 1c provided only small amounts of protodesilylated product 4, as observed by GC/MS. The formation of 4 was not observed for larger substrates 1d and 1e. In contrast, the TMS group was unstable to even 1 equiv of CF3CO2H, and 4 was the only observed benzannulation product. However, 1a was smoothly benzannulated in the presence of ZnCl2 to provide 3a in good isolated yield (75%). The substrate scope of the benzannulation reaction was also expanded beyond phenylacetylene by evaluating a series of aromatic systems containing TIPS-protected acetylenes (Table 2).
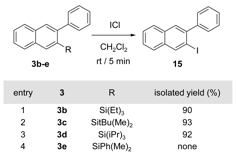
The benzannulation of these model silyl-protected phenyl acetylene substrates provides naphthalene building blocks with variable silyl and aryl groups at the 2- and 3-positions, respectively. Although new C-C bonds may be formed at silyl sites directly under Hiyama cross-coupling conditions, we explored converting the silyl group to a halide to take advantage of a full range of available transition metal-catalyzed C-C, C-N, C-O, and C-F bond-forming reactions. Silyl groups of each of the isolated naphthalenes 3b-d underwent rapid and quantitative conversion to2-iodo-3-phenylnaphthalene 15 upon treatment with ICl in CH2Cl2 (Table 3).
Table 2. Benzannulation of various TIPS-protected acetylenes. Alkyne: (0.05 g) 2: (2 equiv), Cu(OTf)2: (0.05 equiv), CF3CO2H: (1 equiv), 30 min, 100 °C
Table 3. The iodization/protodesilylation of each silyl naphthalane
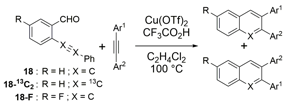
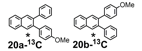
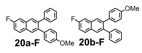
The remarkable efficiency of the benzannulation reaction also warrants elaboration to functionalized benzaldehyde cycloaddition partners. These reactants will enable the synthesis of PAHs with otherwise unavailable substitution patterns, yet introduce the possibility of forming regioisomers. Excellent regioselectivity is paramount, as reaction's most desirable attribute, "its ability to modify polyfunctional alkyne substrates, "would be rendered irrelevant if mixtures of regioisomers were produced. We evaluated the regioselectivity of the benzannulation reaction (Scheme 2) using 18-13C2 and 18-F. Single regioisomers are obtained for many diarylalkynes, including polyfunctional alkyne substrates that serve as desirable PAH precursors. The combinations of aryl substituents studied indicate that electronic effects determine the regioselectivity.
Scheme 2. 13C-labeled and F-substituted derivatives of 18 were used to probe the regioselectivity of the Asao-Yamamoto benzannulation of diarylalkynes, which is non-trivial when Ar1 - Ar2.
The regioselectivity of the benzannulation reaction was first established for
Table 4. The regioselectivity and isolated yields obtained for the benzannulations of various diarylacetylenes by 18-13C2 and 18-F.
substrate |
product(s) |
ratio |
>99:1 (78%) |
||
>99:1 (63%) |
||
49:51 (95%) |
||
49:51 (82%) |
||
>99:1 (99%) |
||
>99:1 (97%) |
||
32:68 (99%) |
||
55:45 (60%) |
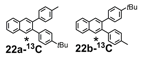
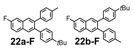
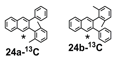
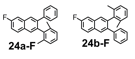
We selected additional substrates 23, 25, 27, and diyne 29 (Table 4; Scheme 3) to evaluate these electronic effects more thoroughly and to determine if steric factors also influence the regioselectivity. Each substrate was benzannulated with 18, as well as its isotopically labeled analogue 18-13C2 and/or fluorine-substituted 18-F. Compound 23 provides insight into the effect of steric hindrance on the benzannulation's efficiency and regioselectivity. 23 was benzannulated efficiently with 18, 18-13C2, and 18-F and provided single regioisomers for the latter two cycloaddition partners, 24a-13C and 24a-F, respectively, which is consistent with the interpretation that the electron-donating property of the methyl groups, rather than their steric demands, direct the cycloaddition. Overall, these observations of the regiochemical outcome and reaction efficiency form the basis of an intuitive model for predicting the products of benzannulation reactions and designing syntheses that are likely to provide naphthalene-containing systems as single regioisomers (see below). Complete regioselectivity is expected from diaryl alkyne substrates that preferentially stabilize positive charge on one alkyne carbon relative to the other.
In summary we have demonstrated that diaryl acetylenes that stabilize developing positive charge preferentially at one alkyne carbon undergo regioselective Asao-Yamamoto benzannulations. The reaction outcome may be predicted without considering steric factors, suggesting that they play little role in determining regioselectivity. Regioselective cycloadditions provide rapid access to precursors of novel polycyclic aromatic hydrocarbons with predetermined substitution patterns and eliminate the need for symmetric substrates in planning complex syntheses. We have also broadened the scope of the benzannulation reaction to silyl-protected phenylacetylenes. When the silyl group is retained, it may be transformed to an iodide under mild conditions to enable further elaboration using various transition metal-catalyzed cross-coupling reactions.