Reports: ND952025-ND9: Activation of Chemical Reactions Through Corona Discharge
Alexandre Yokochi, PhD, Oregon State University
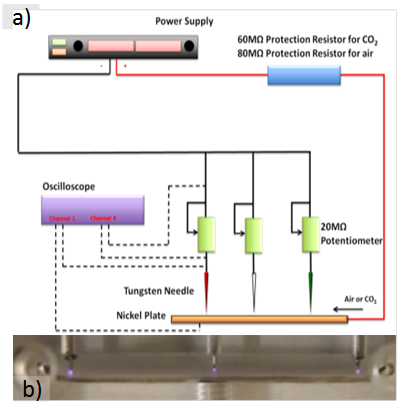
Figure 1. a) Diagram of a multi-emitter reactor and b) a photograph of three simultaneous discharges in the reactor.
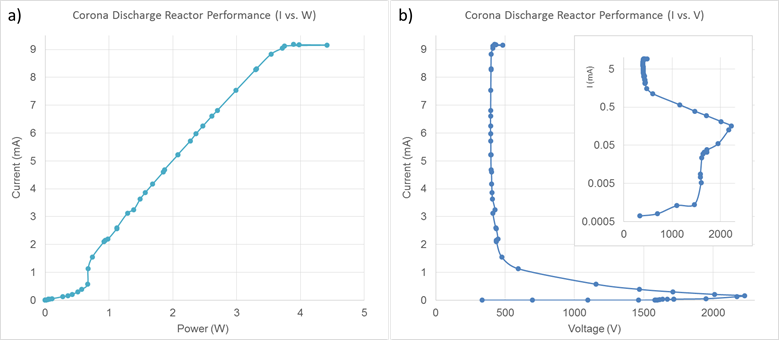
Figure 2. a) Reactor current and b) the resulting Current-Voltage performance of a microstructured corona discharge reactor. Insert shows current on a logarithmic scale.
Figure 3. a) and b) glow discharges and c) arc discharge for a 1mm gap reactor.
Figure 4. A (Trichel) pulse discharge through decane/DBT solution.
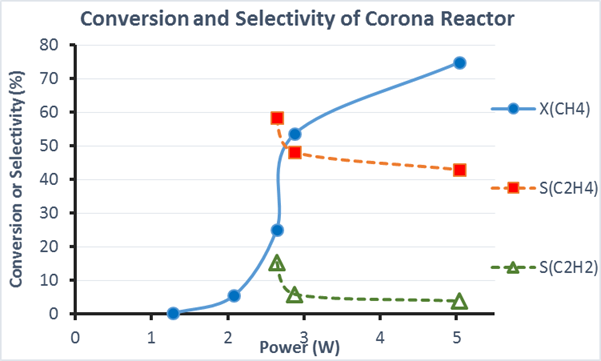
Figure 5. Conversion of Methane (Circles) and selectivity towards ethylene (filled squares) and acetylene (open triangles) as a function of applied power to a multi-emitter reactor.