Reports: ND551762-ND5: Toward Improved Heterogeneous Catalysts for Olefin Metathesis
Bruce E. Koel, Princeton University
A new UHV instrument combining high-resolution XPS (HRXPS), low energy ion scattering (LEIS), high-resolution electron energy loss spectroscopy (HREELS), low energy electron diffraction (LEED), and temperature programmed desorption (TPD), which was installed and commissioned during the prior grant period, was used in this work. HREELS was used for measuring vibrational and optical excitations and LEIS provides the capability to determine elemental concentrations in the topmost atomic layer of samples, complementary to the near-surface concentration and chemical state information provided by HRXPS.
Al2O3 films with a thickness of 5 Å were grown on a NiAl(110) single crystal by exposure of the NiAl(110) surface at 600 K to 1200 L O2 and subsequent annealing to 1200 K. This led to an exceptionally well-ordered Al2O3 film surface, as shown in Figure 1. HRXPS spectra of the Al2O3/NiAl(110) substrate obtained at normal and grazing emission revealed features assigned to the oxide film, the NiAl(110) substrate, and an Al interface layer between the oxide and metal. HREELS showed three primary single loss peaks at 430, 612(658), and 868 cm-1, consistent with previous studies favoring a film structure similar to γ-Al2O3.
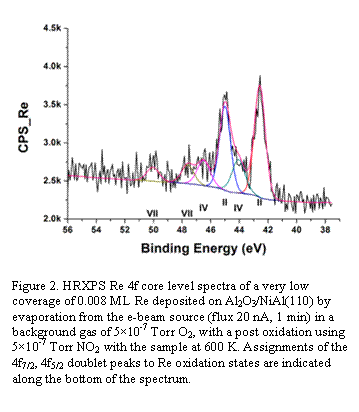
Of central importance to this project was the ability to evaporate and co-evaporate small and medium quantities of refractory metals such as Re, Mo, and W at temperatures of up to >3100 K. During the prior grant period we installed and tested a new evaporator that was specially designed for use in UHV to deposit high purity thin films. A built-in flux monitor allowed for highly controllable deposition rates to form the submonolayer deposits needed for isolated Re, Mo, and W species. Our first studies utilized Re deposition on Al2O3/NiAl(110) substrates. Evaporation of Re for 1 min (20 nA flux) in a background gas of O2 at 5x10-7 torr deposited 0.008 ML Re. We demonstrated that we could detect this small amount of Re using HRXPS. As shown in Figure 2, HRXPS of the Re 4f7/2, 4f5/2 doublet peaks was used to identify the Re oxidation states present on the surface. Assignments of these peaks to Re oxidation states of Re(II), Re(IV), and Re(VII) are indicated along the bottom of the spectrum, with most of the Re existing as Re(II). Larger evaporation times of Re led to deposition of clusters with increasing metallic Re(0) composition, which are not of interest for this project. We also demonstrated that vibrational spectra from HREELS, which can provide key information on the structure of RexOy species on the alumina support, could be obtained from such small surface concentrations and we observed new loss peaks, most notably at 920 and 990 cm-1, for the same surface as in Figure 2. However, HREELS from such mixed surfaces is complicated, and we are seeking methods to produce more well-controlled single species. We are now exploring the utility of using temperature and NO2 exposure to control the oxidation state of Re on the surface for use in further studies. This will enable us to make progress toward our ultimate goal to improve the understanding at the molecular level of the catalytic active sites, their activation and deactivation, reaction intermediates, and the overall reaction mechanism of heterogeneous olefin metathesis over supported metal oxide catalysts.
In conclusion, this project provided fundamental proof-of-concept results, which will serve as a basis for a proposal in this area and position our group to continue research in this new direction. This project provided support and training of one graduate student and two postdoctoral research associates (not fully supported by ACS PRF). These research activities have also enhanced the education of other students and postdocs in our research group through presentations and discussions of these results in several forums involving oral and poster presentations.
The postdoctoral research associate who was supported by these ACS-PRF funds and carried out this work presented her results at the AIChE National Meeting, Atlanta, GA, November 2014 meeting, and we anticipate submitting a manuscript for publication in the coming year.