Reports: UR250759-UR2: Experimental Synthesis of Authigenic Clay Minerals: Implications for Lacustrine Deposition and Diagenesis
Daniel Deocampo, Ph.D., P.G., Georgia State University
This project is using lab synthesis experiments to test several hypotheses based on detailed studies of clay minerals in saline, alkaline lakes in East Africa and other regions of the world (Deocampo, 2004). In these settings, magnesium enrichment observed in ancient lake sediments has been used as an indicator of aridity in ancient times. The recent development of high quality geochronologically controlled stratigraphic sections allows these geochemical indicators to be used in developing robust paleoenvironmental and paleoclimate change reconstructions (Deocampo et al., 2009). In 2013-2014, several major new drilled cores have been obtained by international research teams, notably the Hominin Sites and Paleolakes Drilling Project (http://hspdp.asu.edu) and the Olorgesailie Drilling Project (http://humanorigins.si.edu/research/east-african-research/drilling). Interpretation of the geochemical records of these historic cores will rely in part on experimental perspectives on clay mineral diagenesis. This project therefore will contribute strongly to major international efforts at basin analysis, paleoenvironmental reconstruction, and paleoclimate studies in several East African Rift basins.
Project hypotheses address 1) the role of detrital aluminum-rich substrates on clay authigenisis; 2) aqueous silica saturation state effects on authigenisis; 3) carbonate brines, cation exchange (Ca desorption), and interstratification; 4) biogenic CO2-induced clay dissolution; and 5) the importance of alkali concentrations on low-temperature illitization of Fe-reduced smectite.
To test hypothesis #1, the hydrothermal sepiolite experiments of Wollast et al. (1968) and Mizutani et al. (1991) are being modified by addition of an aluminum-rich detrital component (Clay Minerals Society standard clay SWy-2). To test hypothesis #2, synthetic brines are being formulated with varying silica concentrations, and in contact with buffering diatomite. To test hypothesis #3, simulated brines have been formulated (Jones et al., 1977) to test for cation exchange from calcium-saturated standard clays. To test hypothesis #4, clay suspensions are placed in CO2-enriched waters under ambient and high-pressure conditions.
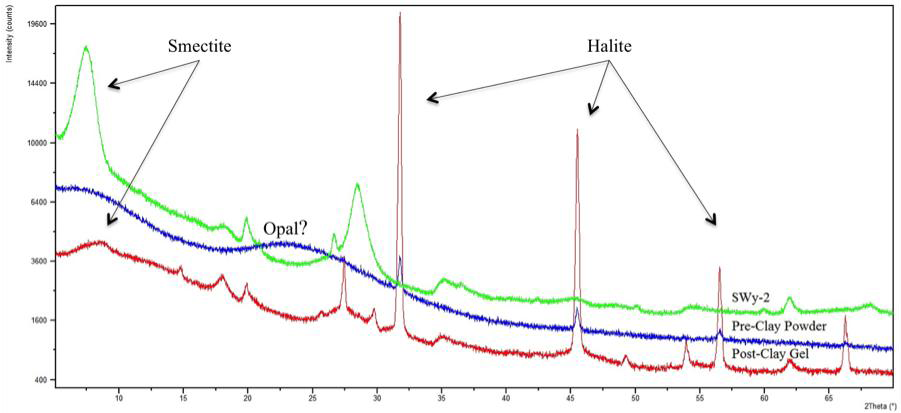
Experimental fluids are being monitored by AA and ICP-AES. Mineralogy is being monitored with a new NSF-supported Panalytical X-ray diffractometer recently acquired by the project PI. Geochemistry of 500mg separates is being determined by Rigaku wavelength dispersive X-ray fluorescence spectroscopy, and SEM and TEM are being used to characterize experimental materials. In Year 3, four undergraduates and one M.S. student were supported by the project. The M.S. student, Rebecca Pickering, defended and completed her M.S. thesis on the project (Pickering, 2014). In her work conducted with the undergraduate assistants, she successfully synthesized trioctahedral domains in magnesium silicate enriched experimental solutions. The students also found that the X-ray diffraction 060 peak in experimental materials, which is associated with the octahedral layer in 2:1 clay minerals, shifted upon exposure to Mg-rich solutions (Figure 1). Whereas detritus-free solutions produced amorphous Mg-silicate gels, the presence of Al-rich detritus led to the development of octahedral structures consistent with a trioctahedral domain in an authigenic clay mineral. Ms. Pickering is now preparing these materials for publication, including the contributing undergraduate students as coauthors.
Figure 1. X-ray diffractogram of SWy-2, Mg-silicate precipitate without addition of SWy-2 ("Pre-Clay Powder"), and precipitate after addition of SWy-2. Slight shift in the 060 peak position suggests reactions in the octahedral layers, perhaps involving neoformation of new trioctahedral domains.
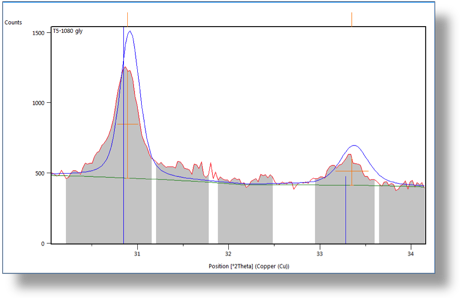
Additionally, one of the undergraduate students had unexpected success in working with magnesium carbonate brines (hypothesis #3). The purpose of the work was to test for cation exchange involving clay minerals and carbonate brines, but the unexpected result appears to be the precipitation of low-temperature protodolomite (Figure 2; Dunham et al., 2014). If we can replicate these results, this is a potentially very important result with implications for understanding the formation of dolomite, particularly in lacustrine environments.
Figure 2. X-ray diffractogram of experimental synthesis products showing the presence of dolomite or protodolomite structures with reflections at 2.89 and 2.68. If confirmed, this would be one of a very small number of successful low-temperature precipitation reactions producing significant amounts of dolomite.
Undergraduate research results will be presented at the upcoming meeting of the Southeast Section of the Geological Society of America. Ms. Pickering has presented her results at the national GSA meeting, and is preparing a manuscript for Deocampo, D.M., 2004. Authigenic clays in East Africa: Regional trends and paleolimnology at the Plio-Pleistocene boundary, Olduvai Gorge, Tanzania. Journal of Paleolimnology, vol. 31, p. 1-9. Deocampo, D.M., Deocampo, D.M. Dunham, J., Deocampo, D., Elrick, K.A., Taylor, L.C., and Pickering, R.A., 2014. The influence of carbonate brines on Ca-Mg exchange and subsequent CaCO3 precipitation. Geological Society of America Abstracts with Programs, vol. 46, p. 100. Jones, B.F., Eugster, H.P., and Rettig, S.L., 1977. Hydrochemistry of the Lake Magadi basin, Kenya. Geochimica et Cosmochimica Acta, vol. 41, p. 53-72. Mizutani, T., Fukushima, Y., Okada, A., and Pickering, R. 2014. Tri-Octahedral Domains and Crystallinity in Synthetic Clays: Implications for Lacustrine Paleoenvironmental Reconstruction. M.S. Thesis, Georgia State University, 120 p.
Wollast, R., Mackenzie, F.T., and Bricker, O.P., 1968. Experimental precipitation and genesis of sepiolite at earth-surface conditions. American Mineralogist, vol. 53, p. 1645-1662.