Reports: UNI153245-UNI1: The Use of Terminally-Functionalized, Atactic- Polypropylene Oligomers as Supports for Homogeneous Catalysis
Christopher E. Hobbs, PhD, Texas A&M University-Kingsville

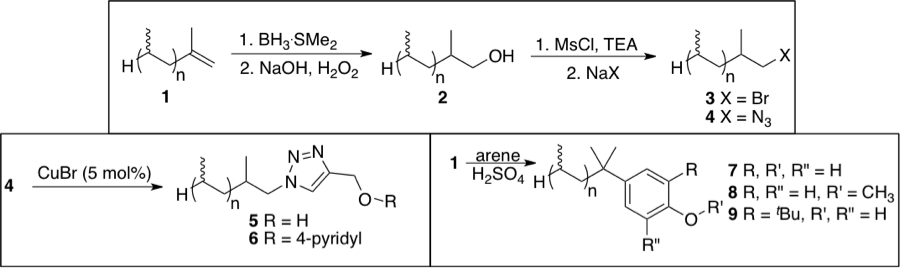
As the title of the grant suggests, the goal of the Hobbs research laboratory has been the utilization of soluble polypropylene (PP) as a new support for synthesis and catalysis. We have shown that this olefin-terminated oligomer can be used as a competent synthetic scaffold for a variety of organic transformations. Some examples are shown in Scheme 1. This material could be subjected to reaction conditions typically discussed in sophomore organic chemistry courses. These include: hydroboration/oxidations and substitution reactions (nucleophilic and aromatic) to provide a variety of functional polypropylenes that have potential as supports for reagents or homogeneous catalysts. Furthermore, this material was shown to exhibit high phase selective solubility for nonpolar solvents (i.e. heptane and hexane), suggesting that this material will be useful as a catalyst handle under liquid/liquid separations.
Scheme 1. Syntheses of PP derivatives.
However, upon the awarding of the grant, the supply of this material was exhausted. And our laboratory was and is not currently equipped to for polyolefin production. This oligomer was originally obtained as a gift from Baker-Hughes, but this supplier discontinued its production. Our laboratory was able to obtain a series of other polypropylene-based oligomers though. Our recent focus has been on the utilization of isotactic-poly(propylene-co-hexene) (iPPH) as a new, recoverable catalyst support.
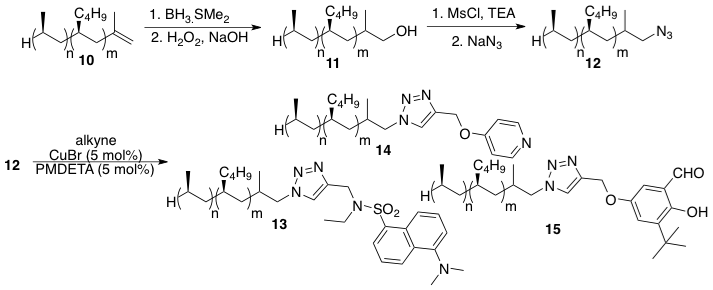
Scheme 2. Syntheses of iPPH derivatives
Like PP, iPPH was obtained from commercial sources (Baker-Hughes) and consists of an olefin end group. This allowed for the preparation of a variety of functionalized copolymers. Much like the chemistry described for PP, this oligomer could be subjected to typical organic reactions such as hydroboration/oxidations and substitutions (Scheme 2). Since our goal falls under the realm of green chemistry, our interests rely on the use of atomic economical click reactions for polymer functionalization. In this case, iPPH-terminated azide could be subjected to reactions with various terminal alkynes under copper-catalyzed coupling reactions. This led to a variety of functional iPPH's, some of which have potential as supported reagents and catalysts.
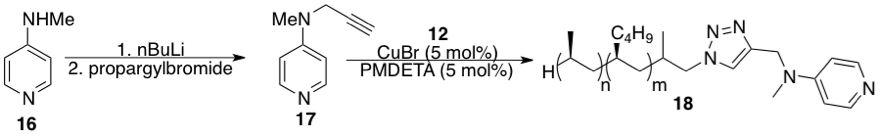
Scheme 3. Synthesis of iPPH-supported DMAP organocatalyst 18
Since the ultimate goal of this project was to develop polymer-supported catalyst/reagents, we attempted to prepare an iPPH-supported organocatalyst (Scheme 3). 4-(N,N-dimethylamino) pyridine (DMAP) and its derivatives can serve as competent organic bases and organocatalysts in a number of reactions. Because of its simplicity and broad usefulness, we were interested in preparing an iPPH-supported DMAP. The preparation of this species was carried out through a copper-catalyzed click reaction between iPPH azide and alkyne-containing DMAP derivative 17. However, in order to synthesize this supported catalyst, the preparation of XX must first had to be carried out. Subjecting 4-(N-methylamino)pyridine (MAP) 16 to a low-temperature deprotonation with nBuLi, followed by reaction with propargyl bromide led to "clickable" DMAP 17 in good yield. Importantly, this compound could be used with no further purification. Reaction of iPPH azide with 18 in the presence of CuBr and PMDETA led to formation of iPPH-supported DMAP.
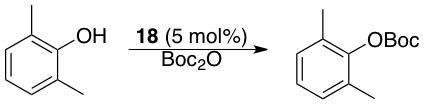
Scheme 4. Use of 18 as a supported organocatalyst
The utility of iPPH as a catalyst support was evaluated through a relatively simple Boc-protection of a hindered phenol, a common DMAP-catalyzed reaction. Advantageously, iPPH-DMAP served as a competent organocatalyst for this process and, unlike many other polymer supports, which are typically only recovered and recycled under solid/liquid or liquid/liquid conditions, this species could be recovered using both. This will allow for iPPH to serve as a one-size-fits-all catalyst support.
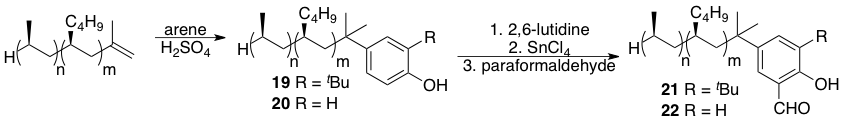
Work currently being pursued in our laboratory is focused on the use of iPPH as a support for other types of catalysts as well. As shown in Scheme 5, we have found that iPPH can be subjected to Friedel-Crafts alkylations. iPPH-supported phenol derivatives 19 and 20 can be converted into supported salicylaldehyde derivatives 21 and 22 which will be useful toward the synthesis of supported salen ligands/catalysts.
Scheme 5. Aromatic Electrophilic Substitutions and formylations to form salicylaldehydes 21 and 22.
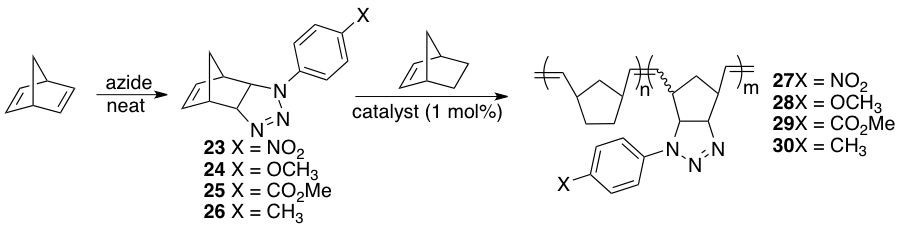
During the course of our studies, we became interested in the functionalization of other types of polymers based on ring opening metathesis polymerization (ROMP) reactions. Typically, ROMP-based polymers' properties can be tuned through the inclusion of various functionalities using either pre-or postpolymerization functionalization reactions. Many of these reactions require transition metal catalysts or excessive amounts of organic solvents, rendering them not sufficiently "green". We have developed a new, click-type process for the construction of new ROMP monomers. This is based off of an old azide-norbornadiene cycloaddition (Scheme 6). Various aromatic azides (23-26) could be subjected to a reaction with excess norbornadiene under solvent free reaction conditions. This has the advantage of generating no solvent waste while providing triazoline products in high yields. Each of these derivatives could be subjected to copolymerizations in the presence of Hoveyda-Grubbs 2ndgeneration catalyst to provide copolymers 27-30.
Scheme 6. ROMP reactions of triazoline-norbornene derivatives.