Reports: ND352242-ND3: Polynuclear Manganese-Oxo Clusters as New Types of Homogeneous Electrocatalysts for Water Oxidation
George Christou, PhD, University of Florida
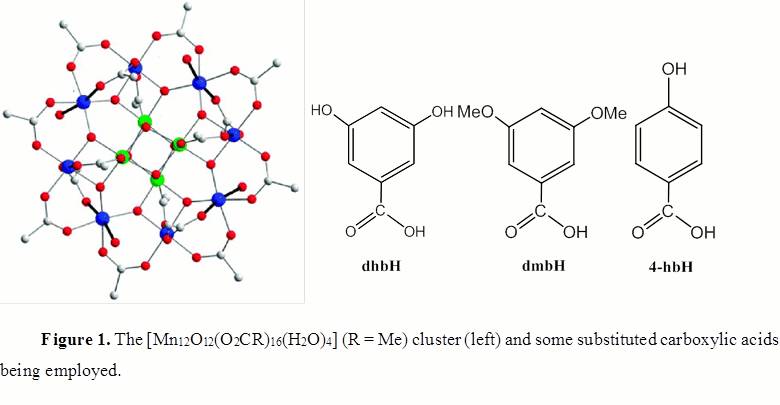
The water solubility and stability was introduced by substituting the 16 acetate groups of [Mn12O12(O2CMe)16(H2O)4], which can readily be prepared in gram quantities, with 3,5-dihydroxybenzoic acid (dhbH). The many –OH groups impart water solubility and the bulky dhb- groups protect the Mn/O core of the molecule from hydrolysis. We have carried out extensive investigation of this compound, and confirmed the reproducibility of its synthesis and properties. We have also confirmed that it is stable for many weeks in aqueous solution, with no apparent change to its solution behavior when studied electrochemically. One question we had was whether it was indeed possible to put 16 bulky dhb- groups around the Mn12 core, and addressed this point by making the related compound with (slightly bulkier) 3,5-dimethoxybenzoate (dmb-) (Fig. 1), which does not have –OH groups, is consequently not hygroscopic like the dhb- cluster, and thus can be better characterized by elemental analysis. This confirmed that the product has completely exchanged all the acetates with dmb- groups, and supports the same situation to have occurred with dhb-. We are planning a complete characterization of the dmb- derivative by X-ray crystallography.
Work during the second year of support has primarily concentrated on extending the synthetic procedures to other Mn-containing clusters to expand the family of available materials for comparative studies of their potential electrocatalytic activity for water oxidation. The concentration of effort on the synthetic side of the project was due to the breakdown of our aging electrochemical equipment and the resulting delay in securing funds for its replacement. The synthetic attempts have met with success and much effort is currently being expended in purifying and characterizing the products and assessing their water solubility and stability.
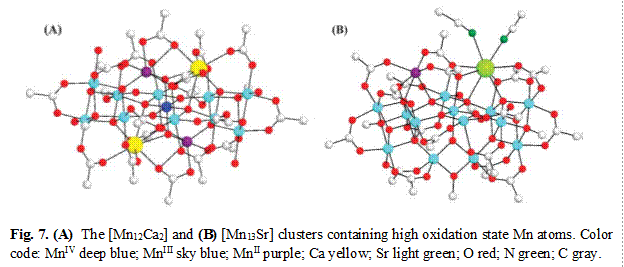
We have recently also extended these efforts to mixed Mn/Ca and Mn/Sr clusters, since the oxygen-evolving complex (OEC) of photosynthesis is a Mn4Ca unit and Sr is the only metal that can replace the Ca with significant retention of water oxidizing ability. We are using the same ligand substitution procedures to introduce hydroxylated carboxylates into these complexes to impart water solubility and stability. The Mn/Ca complex seems to be stable during this procedure, but the Mn/Sr less so, perhaps because of the more accessible Sr ion initiating a structural transformation.v