Reports: DNI152488-DNI1: Formation of Cyano-Carbenes from Alkynes and Azides Using Hypervalent Iodine
Mitchell P. Croatt, PhD, University of North Carolina at Greensboro
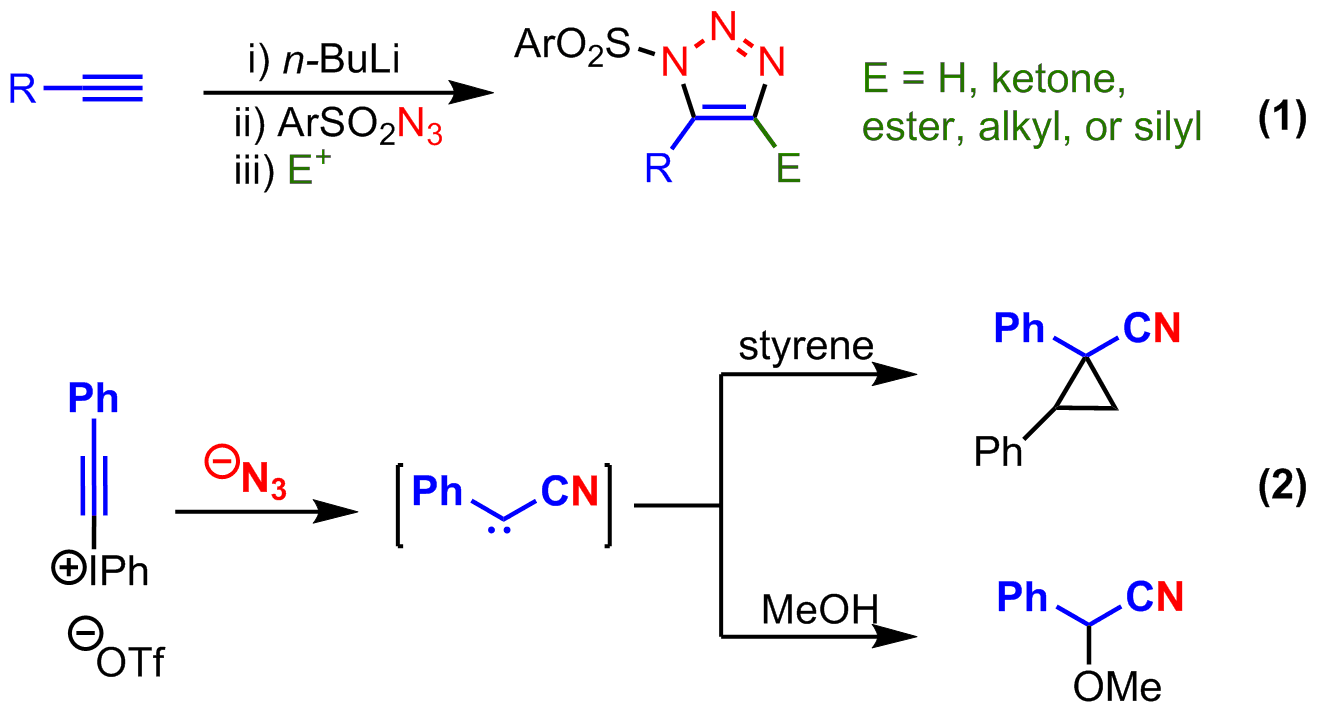
The design and development of new reactions is required for the continued evolution of organic synthesis. Of unique importance are reactions that convert simple, readily available starting materials into products that have significantly increased molecular complexity. With these guiding principles in mind, the research funded by this Doctoral New Investigator award from the American Chemical Society Petroleum Research Fund has been focused on harnessing the inherent reactivity of alkynes and azides. Prior to receiving these ACS PRF funds, my research group reported the reactivity of nucleophilic alkynes with electrophilic azides (equation 1) and electrophilic alkynes with nucleophilic azides (equation 2). The former reaction efficiently yielded 1,5-disubstituted or 1,4,5-trisubstituted sulfonyl-triazoles that had not be previously researched. The latter reaction traversed a variety of reactive intermediates to transiently form a cyanocarbene before reacting with solvent. This latter reaction was the foundation upon which the research was proposed with the ACS PRF grant.
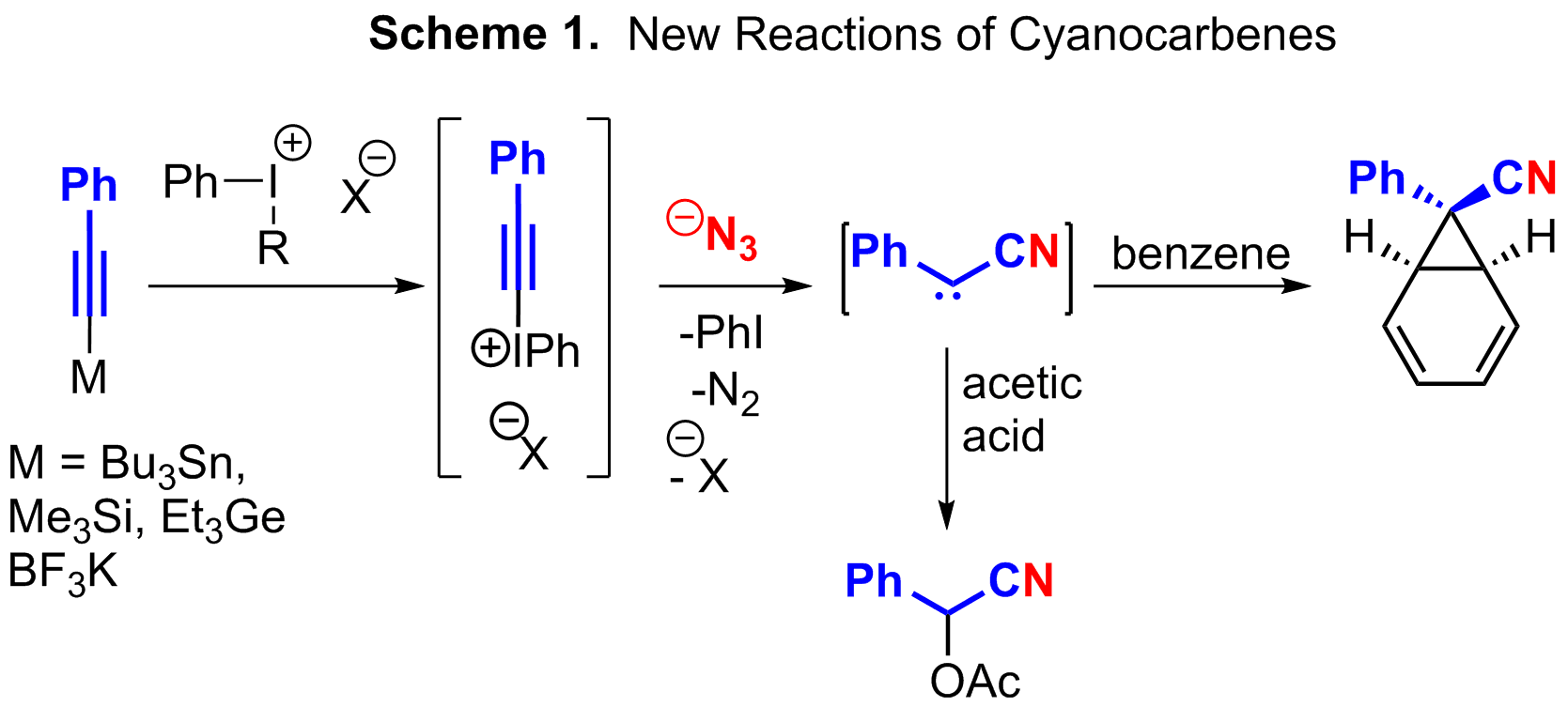
A major hurdle to synthesize cyanocarbenes from electrophilic alkynes and azides is the purification of the hypervalent iodonium alkynyl triflates (HIATs). The HIATs are synthesized efficiently; however, their purification is troublesome due to their moderate to severe air, water, and light sensitivity. In fact, one HIAT needs to be isolated under inert atmosphere at -40 °C. One publication funded by this ACS-PRF in the Journal of Visualized Experiments details this procedure. In order to better solve the isolation difficulties with HIATs, it was decided to optimize a reaction the forms the HIATs in situ and then reacts them with azide to yield cyanocarbene products. As such, we have been able to convert an alkynyl-tin species into a cyanoester where the reaction first forms a HIAT, the HIAT reacts with azide to form a cyanocarbene, and finally the cyanocarbene reacts with acetic acid (Scheme 1). This all occurs in the same reaction flask. This process can also do the same reactions described earlier. In addition to the reaction with acetic acid, cyclopropanation of benzene has been observed. In this case, a single diastereomer is formed, presumably due to equilibration via the cycloheptatriene.
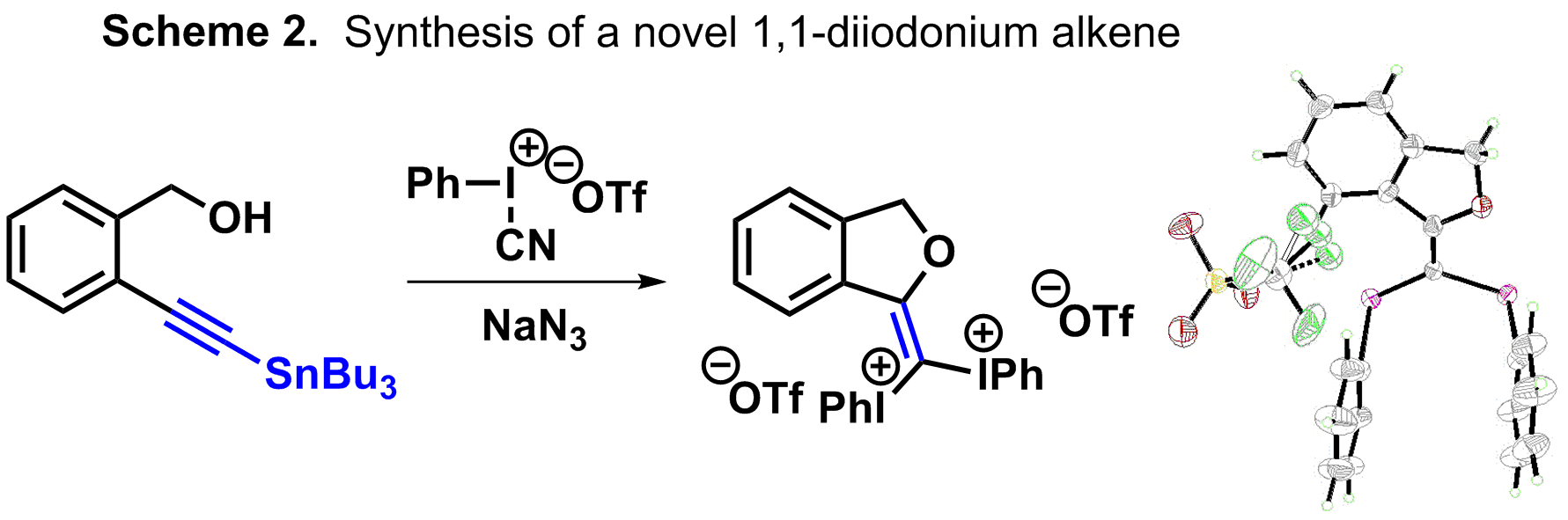
Another advance that is being made involves the alkynyl metal species that is being used. Since tin species are both toxic and relatively labile, alkynyl germanium, silicon, and boron groups have been used. Depending on the source of hypervalent iodine, these alternative sources of the alkyne are more or less feasible. For example, Zefirov’s reagent is reactive enough to form the HIAT from any of the alkynyl metal species, but cyanophenyliodonium triflate requires a tin reagent. This work, funded by this ACS-PRF, has been published in Chemical Communications. In this article we also present some of our results using transition metals to form the cyanocarbenes without traversing vinylidene carbenes and the unexpected formation and characterization of the first 1,1-diiodonium alkene (Scheme 2).
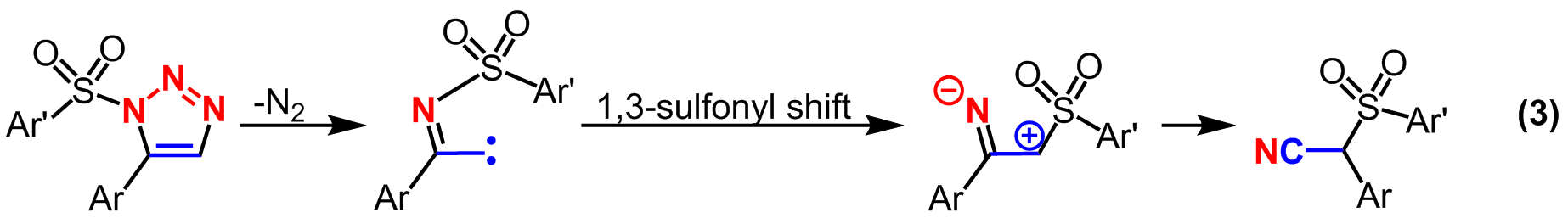
The original goal of the triazole research was to actually form cyanocarbenes as detailed in our recent SYNPACTS article in Synlett. While not giving up on making carbenes in the triazole project, it was decided to thermalize the products. In doing so, it was determined that the 1,5-disubstituted triazoles that our group previously synthesized behaved quite differently compared to the commonly observed 1,4-disubstituted triazoles. With our triazoles, thermolysis extruded dinitrogen to form a carbene that then underwent a sulfonyl migration and eventually formed a series of α–sulfonylnitriles (equation 3). This reactivity was also possible using a Rh(II)-catalyst, however, it appears that the transition metal may be merely acting as a Lewis Acid. The reactions with cyanocarbenes and triazoles both appear to have the intermediacy of a carbene and also form products that result from novel disconnections. In both cases, the nitrile that is formed results from one carbon of the alkyne and one nitrogen of the azide. The retrosynthetic disconnection of a cyano group to an alkyne and azide is abnormal, and therefore the reactions accomplished from these projects are enabling novel types of reactivity to be envisioned. This work, funded by the ACS-PRF, is published in Tetrahedron.
Due to the generous support of my group’s research from the ACS PRF, we have been able to further develop the methods available to form of cyanocarbenes and study their reactivity. This project grew and developed in previously unanticipated areas. With the funding from the ACS PRF, this project was positioned to be highly competitive for external funding and was successful in obtaining an NSF CAREER Award in 2014. Furthermore, the travel funds from this grant have allowed researchers on this project to give poster presentations at a Regional and National ACS meetings.
Thus far, four publications have resulted from this research and three are added to the ACS PRF web reporting system (one will be entered once page numbers are assigned). These publications strongly benefit the PI, the postdocs on this project, and the undergraduate students that are co-authors of the publications. For the PI, the research obtained and published from the ACS PRF grant were critical to obtaining an NSF CAREER Award. Both of these grants positioned the PI to go up for promotion and tenure a year early. The publications for the postdocs are significant since one is applying for academic positions. The undergraduates that co-authored some of the research have been accepted into graduate programs (Michigan and Florida State) and Medical School (Virginia) and these publications helped to set them apart from other applicants.