Reports: ND750941-ND7: Tetrazines for Hydrogen Storage
Douglas A. Loy, PhD, University of Arizona
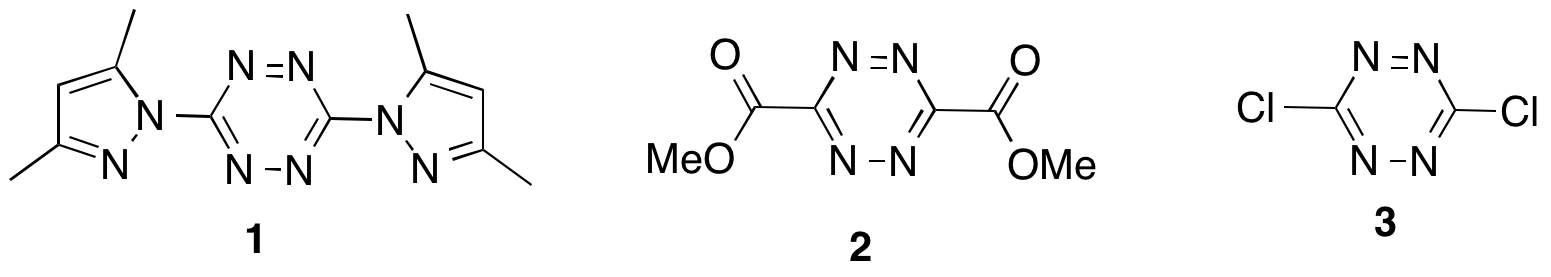
The goals of the project were to synthesize tetrazine monomers, such as the bis(dimethylpyrazole)tetrazine (1) or dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate (2) or 3,6-dichloro-1,2,4,5-tetrazine (3) prepare polymers either with the tetrazine moieties in the polymer backbone or as pendant functionalities, and explore the chemistry of the polymers, including the hydrogenation/dehydrogenation chemistry of the tetrazine ring system. What we discovered was a rich field of chemistry and materials based on the highly colored tetrazines with numerous potential applications, including self-developing photoresists, new classes of thermosets with color indicators, and more.
Monomer synthesis
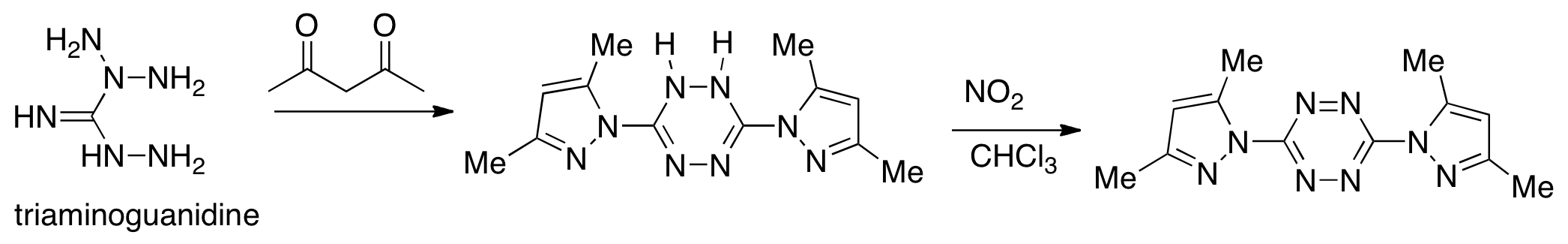
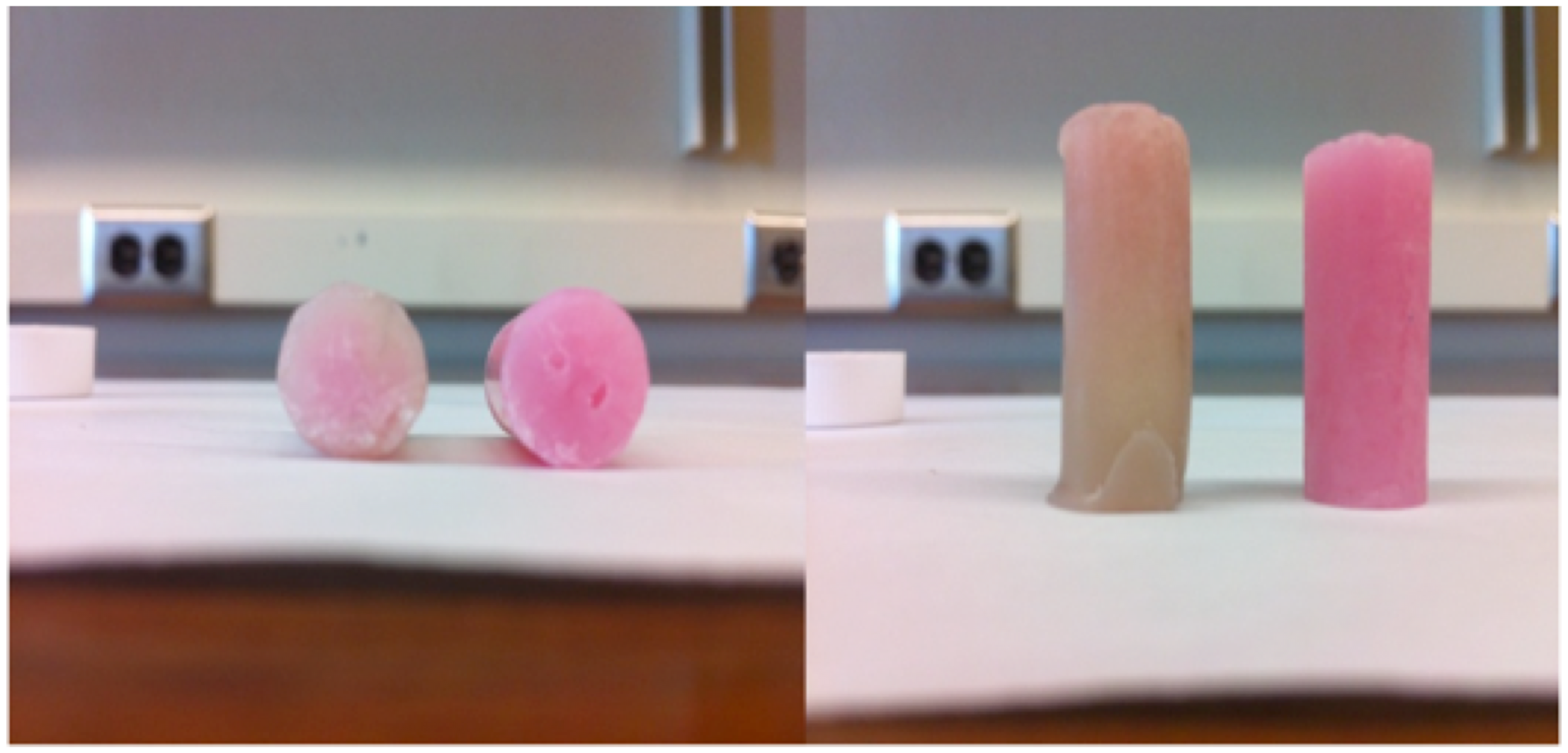
Synthesis of tetrazine 1 is shown in scheme 1. The dihydrotetrazine oxidized to 1 upon standing in air. Due to its extended conjugation, 1 was found to be a striking red hue (Figure 2), a characteristic typical for these heterocyclic molecules. The hydrogenated tetrazines were pale yellow (Figure 1).
Scheme 1. Synthesis of tetrazine monomer 1
Figure 1. 3,6-bis(3,5-dimethyl-1H-pyrazol-1-yl)-s-tetrazine (1)
Tetrazine hydrogenations
Tetrazines were hydrogenated with one atmosphere of hydrogen in the presence of chloroplatinic acid. Upon addition of two hydrogens, the compounds decolorized to a pale white appearance similar to the dihydrotetrazine intermediate in the synthesis of 1 (Figure 2).
Figure 2. 3,6-bis(3,5-dimethylpyrazol-1-yl)-1,2-dihydro-1,2,4,5-tetrazine
The progress of the hydrogenation could be readily followed with
Tetrazine solutions in polymers
If the tetrazines can be dissolved in polymers, particularly those with glass transition temperatures lower than room temperature, realtively simple hydrogen storage systems could be prepared more readily and potentially with greater quantitites of hydrogen. To this end, we examined the solubility of the tetrazines in molten polymers and in polymers already dissolved in solvents known to dissolve tetrazines. However, while tetrazines were soluble at elevated temperatures, the materials crystallized out with cooling to room temperture. Solutions of tetrazines could be made in polystyrene ad polyethylene oxides, Only the latter demonstrated decolorization with exposure to hydrogen.
Figure 3: Hydrogenation of tetrazines in polyethylene oxide using chloroplatinic acid as the catalyst.
However, the rates of hydrogenation were generally too slow (hours) to provide effective hydrogen storage. Reoxidation of the reduced tetrazines back to their highly colored aromatic versions proceeds quickly in solution, but were slow in solid state polymers.
Polymerization experiments
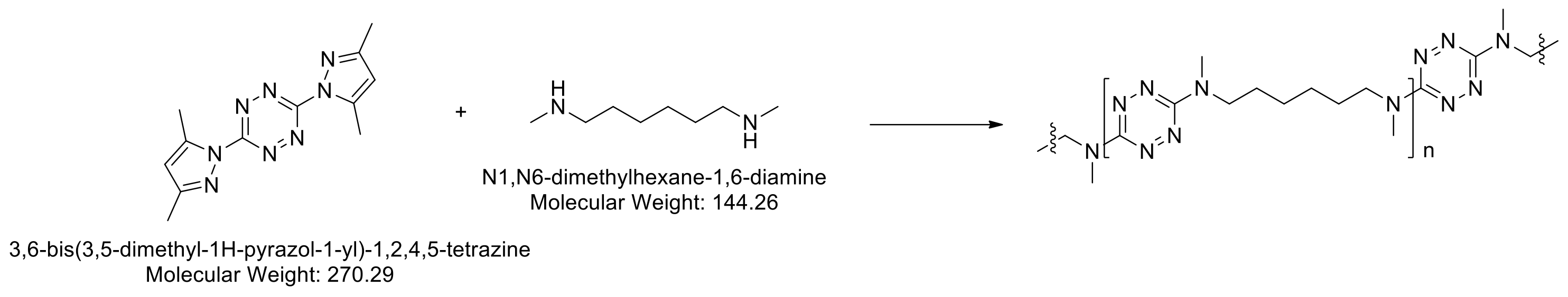
Polymerization of the tetrazines occurs by nucleophilic substitution of the dimethylpyrazole groups from the 3 and 6 positions on the aromatic ring. Polymerization of 1 with 1,2-diaminoethane or 1,6-diaminohexane resulted in insoluble, crosslinked tetrazine based polymers. We discovered that it was possible to copolymerize 1 with bis-amine monomers to afford linear soluble polymers (Scheme 2).
Scheme 2. Polymerization of tetrazine monomer 1 with diamine co-monomer
While it was discovered that 1 did not polymerize with diol or dithiol comonomers, 3,6-dichlorotetrazine 3 did copolymerize to form linear oligomers and polymers. The copolymer of 3 with ethane dithiol is of particular interest as we can oxidize the sulfide groups in the polymer backbone to the sulfones that are succeptible to photochemical degradation. We are presently evaluating the polymers UV stability and the potential utility of these materials as self-developing photoresists.
Modification of polymers with pendant tetrazine groups
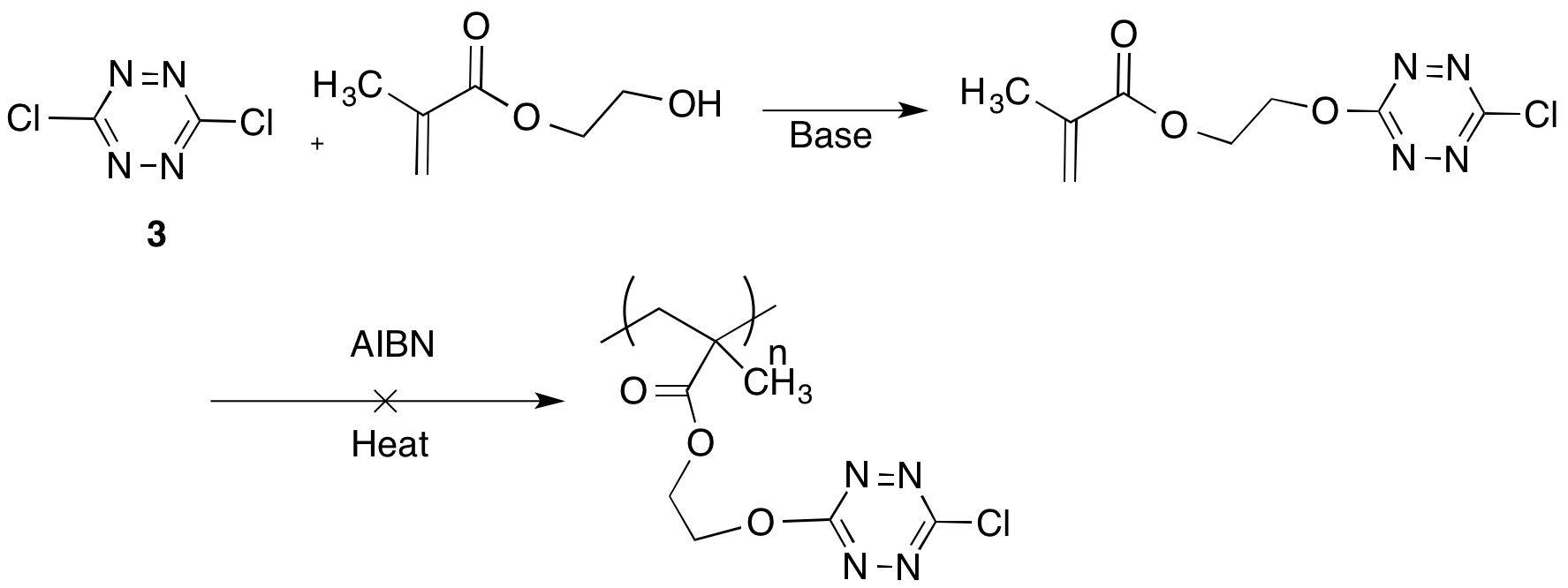
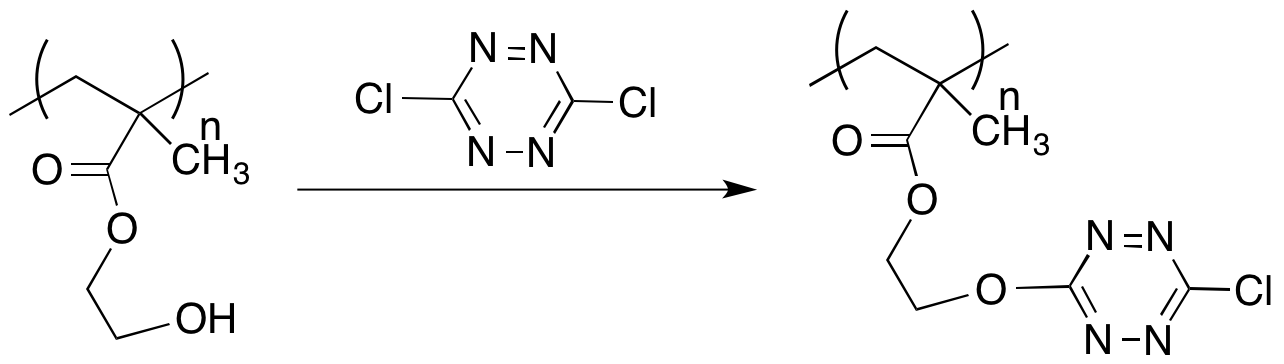
Polymers with pendant tetrazines were prepared by either modifying a monomer with a pendant tetrazine group or by post-polymerization modification of a polymer. A methacrylate monomer was prepared from hydroxyethyl methacylate, but the monomer appeared to inhibit free radical polymerizations (Scheme 3). A more expedient approach to preparing modified polymers was to react the dichlorodtetrazine with polymers with pendant nucleophiles. Poly(hydroxyethyl methacrylate) was successfully reacted with the dichlorotetrazine to afford a bright red polymer (Scheme 4). The polymer could then be reacted with dienophiles by Diels Alder cycloadditions to generate additional new polymers or with bis-dienes to irreversibly crosslink the thermoplastic.
Scheme 3. Formation of acrylate monomer.
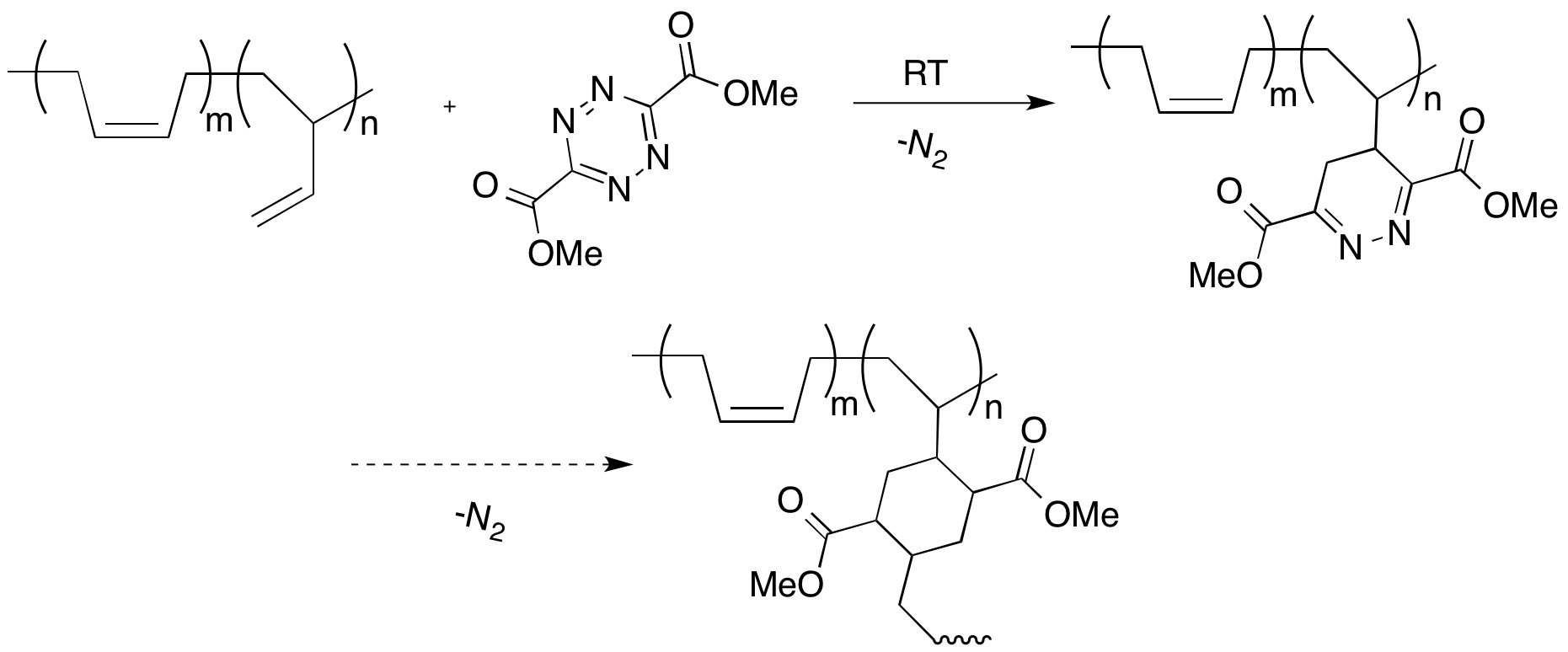
We also discovered that the tetrazines would undergo Diels Alder cycloadditions with liquid, low molecular weight polybutadiene (Scheme 5). The Diels Alder reaction and subsequent retro Diels Alder release of molecular nitrogen occur at room temperature. The reaction can be monitored by the slow change in color from intense red to yellow and by the release of bubbles of nitrogen gas. We are currently studying if the resulting polymer will cross-link through a second Diels Alder cycloaddition and if the reaction can be used to generate foams.
Scheme 4. Modification of pre-formed polymethacrylates.
Scheme 5. Reaction of 2 with polybutadiene.
Diels-Alder Chemistry of Tetrazines.
Tetrazines hae been shown to react with dienophiles in [4 + 2] cycloaddition reactions with subsequent [4 +2] retro-Diels-Alder elimination of dinitrogen. We have studied the reactivity of tetrazines with different functional groups and the reactivity of the tetrazine as a diene relative to the pyridazine (diazabenzene). Theoretically tetrazines should be able to undergo two Diels-Alder cycloadditions and dinitrogen elimination reactions to form the nitrogen free aromatic product. This would allow tetrazines to be used as monomers in Diels-Alder polymerization reactions leading to polyarylenes. Model studies began late in the project and are continuing to date.
Conclusions
The project afforded a new family of polymeric materials based on tetrazine functional groups. This includes copolymers based on diamino- dithiol and dihydroxy functionalized comonomers. We have demonstrated hydrogenation and dehydrogenation of tetrazine functionalities in low glass transition temperature polymers, but have concluded that the rates in these systems are too slow to be useful for fuel storage. We have also shown that the polymers prepared from ethane dithiol can be oxidized to the polyemr with sulfones in the backbone, a potential new class of self-developing photoresists. Future hydrogen storage efforts will focus on improving hydrogen transport by incorporating the tetrazine functionalities into gels made from polymer and solvent. Diels-Alder polymerization chemistry of tetrazines will be used to prepare new polyarylenes and to crosslink new thermoset materials.