Reports: ND352988-ND3: Aerobic Oxidative C-C and C-Heteroatom Bond Formation Reactions Catalyzed by Novel Pd(III) and Pd(IV) Complexes
Liviu M. Mirica, PhD, Washington University
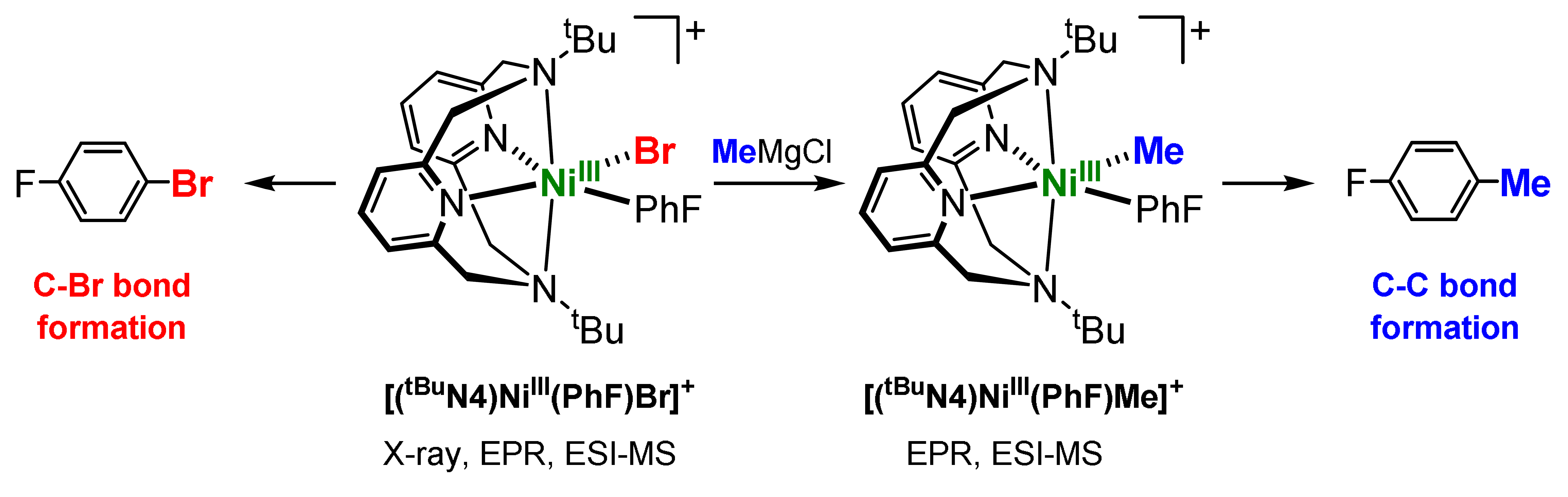
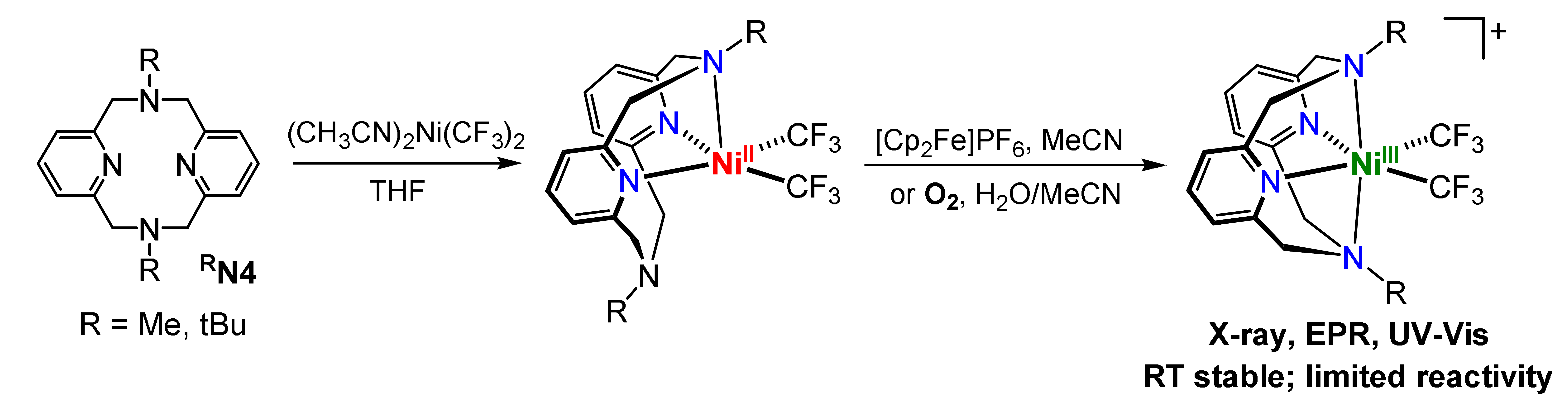
Scheme 1 While a NiIII(aryl)alkyl intermediate was spectroscopically characterized in the study mentioned above, that complex undergoes rapid reductive elimination and could not be isolated. In order to further stabilize such organometallic NiIII complexes, we hypothesized that the use of perfluoroakyl ligands will slow down the reductive elimination reactivity of these species. Such an approach has been elegantly used recently by Vicic et al. to detect spectroscopically a terpyridine-supported bis(trifluoromethyl)NiIII complex. In this context, we have also recently reported the isolation and detailed characterization of uncommonly stable mononuclear bis(trifluoromethyl)NiIII complexes supported by the pyridinophane ligands MeN4 and tBuN4. Interestingly, these NiIII complexes can be generated upon facile oxidation of the NiII precursors, including aerobic oxidation, and surprisingly they exhibit a very limited reactivity. Overall, these studies strongly suggest the RN4 tetradentate ligand system is capable of stabilizing various bis(hydrocarbyl)NiIII complexes that would allow for their isolation and detailed investigation of their reactivity relevant to cross-coupling reactions.
Scheme 2