Reports: ND1052475-ND10: Structure and Dynamic Studies in Metal-Organic Materials
Tomislav Friscic, PhD, McGill University
Reaction optimization. Optimization of the accelerated aging (AA) synthesis of the new BODIPY@ZIF-8 fluorescent composites revealed that the best results, leading to quantitative formation of the ZIF-8 structure, are obtained by using caffeinium sulfate as a catalytic salt additive.
Mechanochemical synthesis. Whereas the AA approach is highly materials- and energy-efficient, it is also time consuming, requiring 1-2 days for the complete conversion of starting materials. In order to provide a rapid synthesis of the BODIPY@ZIF-8 composites, we developed a mechanochemical approach towards these fluorescent materials. The optimized synthesis consisted of mechanochemical liquid-assisted grinding (LAG) transformation of a mixture of ZnO and HMeIm into ZIF-8, aided by 4 mol% of a salt catalyst (ammonium nitrate) in the presence of a selected amount of the BODIPY dye. Using this procedure, it was possible to obtain materials with BODIPY wight content between 0.05% and 5%, in gram amounts and within 30 minutes. Consistent with the results for the entrapment of BODIPY dyes using accelerated aging, mechanochemically obtained materials also exhibited prnounced solid-state fluorescence and did not lose the dye upon washing with organic solvents.
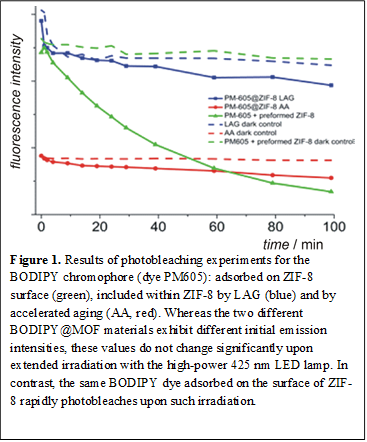
Photostability and photophysics of BODIPY@ZIF-8. Following the previously described activation of solid-state fluorescence in BODIPY@ZIF-8 composites, we established a significant improvement in the solid-state photostability of the dye included in the framework, compared to a dye that is simply adsorbed on the framework surface. Irradiation of the PM605 dye adsorbed on ZIF-8 (made, for example, by milling pre-made ZIF-8 with the dye) with a 425 nm LED lamp led to rapid photobleaching (Figure 1). In contrast, irradiation of the BODIPY@ZIF-8 made by LAG or AA led only to a small initial drop in fluorescence, after which the fluorescence emission changed slightly over several hours. This is an unexpected, new observation in the photochemistry and photophysics of BODIPYs and opens the door to potential new solid materials based on the otherwise highly photosensitive BODIPY chromophore. The fluorescence lifetimes of BODIPY chromophores were also measured for a selection of BODIPY@ZIF-8 materials containing different concentrations of the included PM605 dye. At low concentrations, the dye exhibited a fluorescence lifetime of τ1 of 7.35 ns, which is higher than measured in solution (6.93 ns), consistent with a lower mobility of the chromophore in the solid. The emission decay also revealed another lifetime component τ2 (3.32 ns), indicating two different environments of included BODIPY guests. Fluorescence lifetimes τ1 and τ2 were decreased with increased concentration of the dye, down to τ1=5.50 ns and τ2=2.25 ns for a composite containing 5% dye by weight, consistent with increasing chromophore interactions.
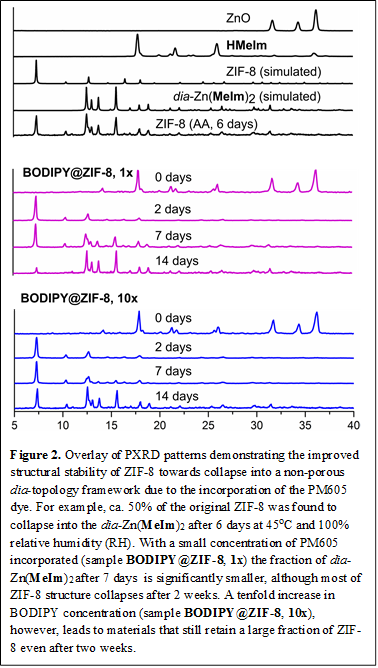
Structural stability and porosity. As established by nitrogen sorption BET analysis, the inclusion of small amounts of a BODIPY dye (e.g. PM-605 or HClBOH dyes) does not significantly affect the high surface area of ZIF-8, which remains > 1000 m2/g. Structural stability of the composites was explored by exposing the materials to mild temperature (45 oC and 100% relative humidity). Surprisingly, the presence of the BODIPY guest enhances the stability of ZIF-8 towards collapse into a non-porous material with the diamondoid (dia) topology, dia-Zn(MeIm)2 (Figure 2).1,2 Consequently, incorporation of low amounts of the BODIPY dye within the porous space of the ZIF-8 framework leads to stable fluorescent materials that exhibit high microporosity, increased chromophore photostability and greater structural stability.
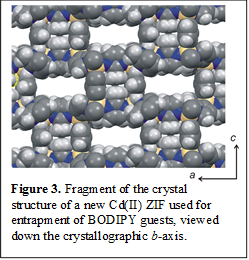
Exploration of new MOF hosts. Investigation of the inclusion of BODIPY dyes was expanded from the commercially important ZIF-8 to new frameworks of higher porosity. In that context, we have found the new open framework based on Cd(II) ions highly promising. This framework, not yet been reported in the literature, provides pores of internal diameter 8 Å, permitting the inclusion of a larger number of chromophoric guests compared to ZIF-8 (Figure 3). X-ray crystallographic studies of the new framework containing the BODIPY guests are currently ongoing.
References:
1) Mottillo, Lu, Pham, Cliffe, Do, Friščić, Green Chem. 2013, 15, 2121-2131; 2) Mottillo, Friščić, Angew Chem. Int. Ed. 2014, 53, 7471-7474.