Reports: UR152993-UR1: Aromatic Donor-Acceptor Organocatalysis: Noncovalent Activation of Aryl Halides in Green Palladium Cross-Coupling Reactions
Joseph J. Reczek, PhD, Denison University
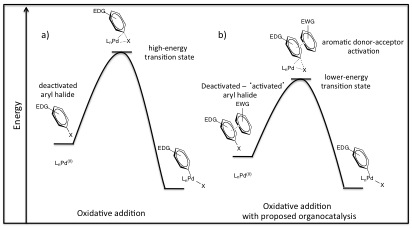
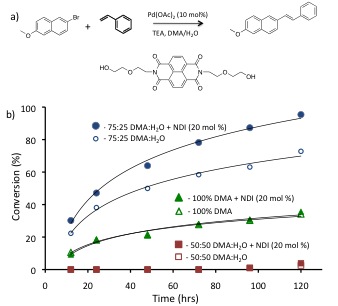
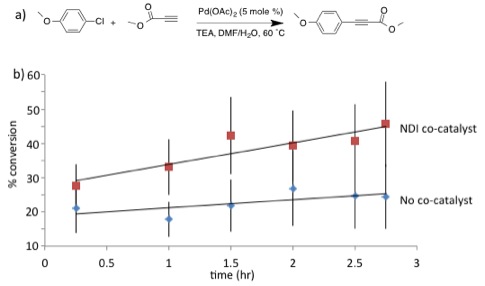
Figure 3. a) Sonogashira coupling reaction initially explored.
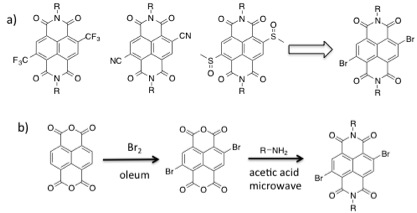
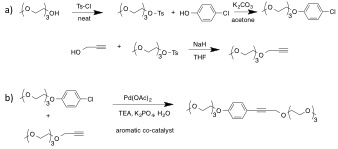
As we continue to develop model reaction set-ups to illustrate general ADA organocatalysis, we have also been working towards the syntheses of electron-poor aromatic catalysts other than NDI.