Reports: ND351715-ND3: A New Method to Link Metal Ions via Cycloaddition of Metal-Azides to Metal-Acetylides
Adam Veige, PhD, University of Florida
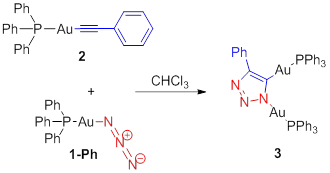
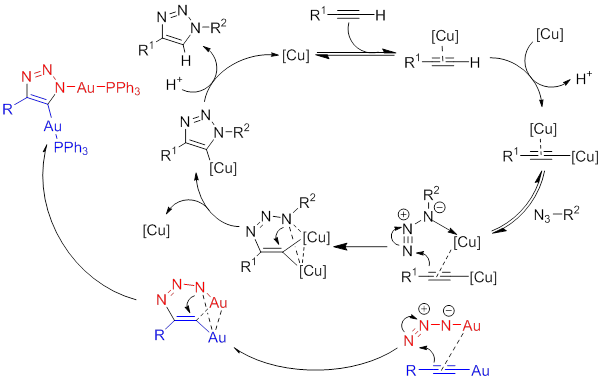
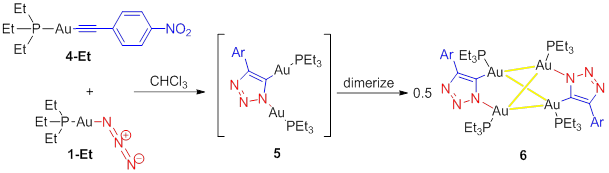
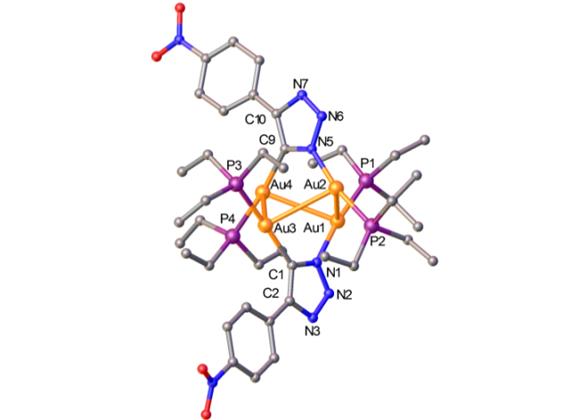
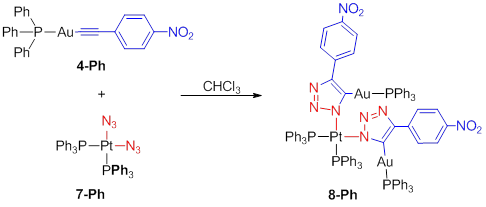
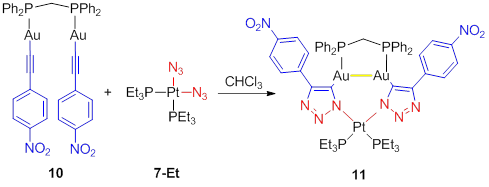
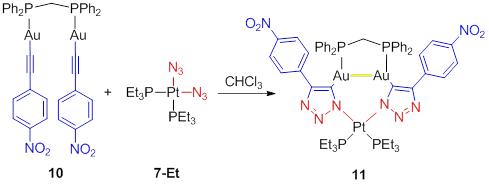
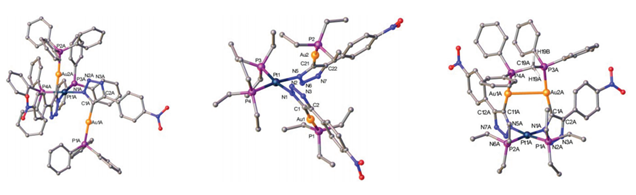
Adam Veige, PhD, University of Florida








Copyright © American Chemical Society