Reports: DNI1052830-DNI10: Synthesis and Electrochemical Characterization of Novel Polyanion Materials for High Capacity Li-Ion Cathodes
Candace K. Chan, PhD, Arizona State University
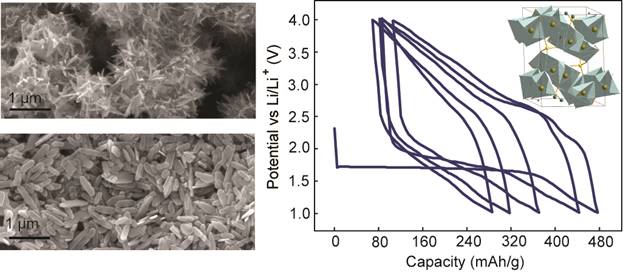

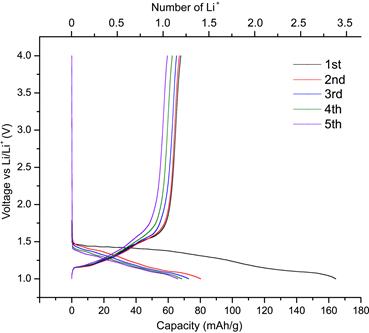
Candace K. Chan, PhD, Arizona State University



Copyright © American Chemical Society