Reports: ND1052056-ND10: Metal Oxide Cathodes and High-Conductivity Ionic Liquids for Next-Generation Batteries
Erik J. Menke, PhD, University of California (Merced)
For the past year we have been investigating the electrochemical behavior of aluminum triflate in diethylene glycol dimethyl ether (diglyme). This work is motivated by work in the previous year demonstrating that the ethyl methyl imidazolium/aluminum chloride ionic liquid is highly corrosive, leading to side reactions with stainless steel current collectors in aluminum-ion batteries. Working under the hypothesis that the high chloride concentration is responsible for the corrosivity of the ionic liquid, we have been testing alternative possible electrolytes, focusing primarily on aluminum triflate in diglyme.
The majority of the work in the past year has been on the physical and electrochemical characterization of the aluminum triflate/diglyme solutions. We have measured how the aluminum to diglyme ratio affects the electrochemical stability, the ionic conductivity, the aluminum, triflate and diglyme diffusion, the vapor pressure and the heat capacity of the electrolyte.
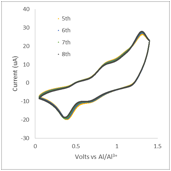
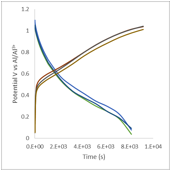
These results have motivated us to test these electrolytes as possible electrolytes for aluminum ion batteries. For the cathode, we have chosen to use a copper-based Prussian blue, and for the anode we use aluminum metal. The battery behavior is summarized in figure 1, below, demonstrating that, for the first time we are aware of, we have prepared a rechargeable aluminum-ion battery. While the battery system has low energy density, primarily due to the low charge capacity of the Prussian blue, and can only be cycled about 20 times before failure, rechargeable aluminum batteries have the potential to be low cost alternatives to lithium-ion batteries.
Figure 1: The left image shows cyclic voltammagrams for a Prussian blue/carbon black electrode in an electrolyte of aluminum triflate in diglyme with a molar ratio of 15:1 diglyme:aluminum, while the right image shows the charge and discharge behavior of a Prussian blue/carbon black electrode in the same electrolyte.
Impact
While this work has so far only led to one publication, it has been presented at the Spring 2014 MRS meeting, the Fall 2014 ACS conference, and the Summer 2014 Electrodeposition GRC. We also have four papers currently in preparation or submitted as a result of this work, with an undergraduate student as first author on one of the papers. In terms of impact on the PI's research, this grant has allowed the group's research efforts to move in a completely new direction. Prior to this grant the majority of the work in the PI's lab was focused on nanowire synthesis for solar cells, while our current research is now equally balanced between the solar research and battery materials. Furthermore, in the last year we have been able to gather enough results to justify research proposals that are being submitted to NSF, DOE, and DoD.
In terms of student impact, this grant has supported two graduate students, one of whom received his master's degree this past summer, and a second who is currently writing his dissertation with plans to defend in December. In addition, an undergraduate student has been working on this project and has presented a poster on this work at the Fall, 2014 ACS conference.