Reports: ND1052588-ND10: Elucidating Competing Transport and Kinetic Mechanisms for Understanding Material Durability of Carbon Felt Electrodes
Venkat R. Subramanian, Washington University in St. Louis
1.
OBJECTIVES:
2.
RESEARCH FINDINGS
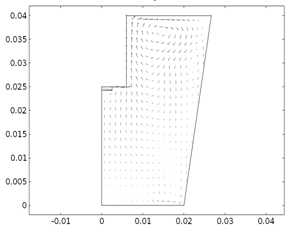
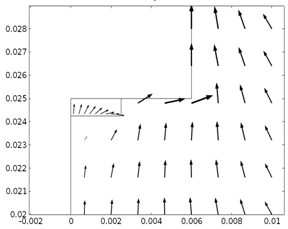
Fig. 1. (a) Arrow plot for
the velocity field in the 2D Porous RDE in both porous electrode and bulk
electrolyte domain at 12,000 rpm (b) Inset of velocity field in the porous felt
region and its vicinity.
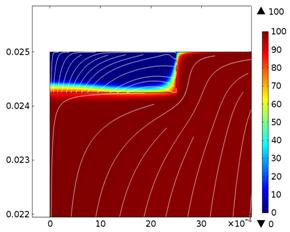
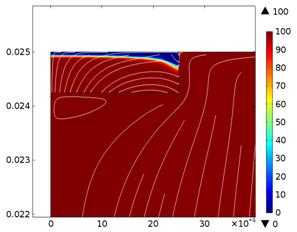
Fig. 2. Spatial variation
of V 3+ species concentration (mol/m3) for low and high rotation
rates with 0.75 mm thick porous felt (A. 634 rpm & B. 3791 rpm)
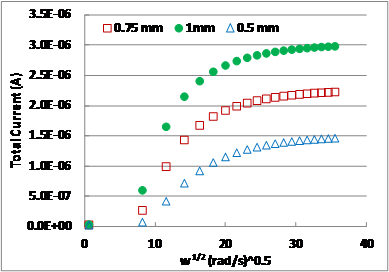
Fig. 3.Variation of total
current in the disk with rotation speed (rpm) as a function of film thickness. 3.
OUTCOMES
4.
FUTURE RESEARCH
2.
T. Nguyen, R.
Savinell, Flow Batteries, in: The Electrochemical Society Interface, vol. 19,
The Electrochemical Society, New Jersey, USA, Fall 2010, pp. 54-56.
![]()