Reports: ND752970-ND7: Dynamics of Confined Polymer Liquids and Glasses under Positive and Negative Pressures
Rodney D. Priestley, PhD, Princeton University
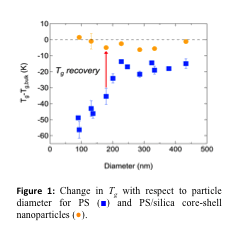
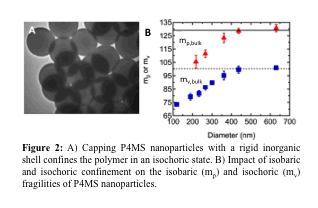
Rapid cooling, the typical route to a glass is accompanied by changes in temperature and volume, both of which contribute to slow dynamics. A potentially powerful study to understanding confined polymers would be to investigate the slow dynamics under isobaric and isochoric confinement conditions in an effort to separate the temperature and volume contributions to glass formation. We developed an approach to quantify the fragility in both isobaric and isochoric conditions; our ability to directly measure the isochoric fragility in either bulk or confinement represents a major achievement. Isochoric confinement was achieved via the capping of polymer nanoparticles with a rigid inorganic shell (Fig. 2a), and fragility was measured via variable-cooling rate DSC using the Moynihan/Angell approach. As illustrated in Figure 2b, both the isobaric and isochoric fragilities decrease with confinement, with a nearly identical onset value for poly(4-methyl styrene) (P4MS). Surprisingly, Tgdecreases and remains constant with isobaric and isochoric confinement, respectively.
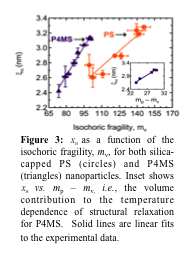
Relating changes in glassy properties under confinement to a fundamental length-scale of glass formation remains a major challenge. A classical theory to explain the rapid increase in cooperative segmental relaxation time as Tg is approached from higher temperatures is the Adam-Gibbs (AG) theory. The central feature of the AG theory is that a growing length scale (xa) is required for cooperative segmental motion or rearrangement, as T is decreased towards Tg. Within the framework of the AG theory, fragility should correlate with both the size of a CRR at Tg, i.e., the characteristic length of the glass transition (xa), and the rate of change in xa, with temperature at Tg. We examined the relationship between xa and mv for polymers under confinement. We found that a linear correlation between xa and mv was observed for silica-capped polymer nanoparticles of PS and P4MS as illustrated in Figure 3 for PS and P4MS. However, as Tg remained constant with confinement for silica-capped NPs, how our results satisfy the AG theory is intriguing; see ref. 3 for more discussion.
1. C. Zhang, Y. Guo and R.D. Priestley,"Glass transition temperature of polymer nanoparticles under soft and hard confinement." Macromolecules 44, 4001 (2011)
2. Y. Guo, C. Zhang, C. Lai, R.D. Priestley, M.D'Acuzni and G. Fytas,"Structural relaxation of poymer nanospheres under soft and hard confinement: Isobaric versus isochroic glass formation." ACS Nano 5, 5365 (2011)
3. C. Zhang, Y. Guo and R.D. Priestley,"Characteristic length of the glass transition in isochorically confined polymer glasses." ACS Macro Letters 3, 501 (2014)
4. C. Zhang and R.D. Priestley,"Fragility and glass transition temperature of polymer confined under isobaric and isochoric conditions." Soft Matter 9, 7076 (2013)
5. C. Zhang, Y. Guo, K. Shepard and R.D. Priestley,"Fragility of an isochorically confined polymer glass." Journal of Physical Chemistry Letters 4, 431 (2013)
6. C. Zhang, Y. Guo and R.D. Priestley,"Confined polymer properties of nanoparticles." Journal of Polymer Science Part B Polymer Physics 51, 574 (2013)
7. C. Zhang, V.M. Boucher, D. Cangialosi and R.D. Priestley,"Mobility and glass transition temperature of polymer nanospheres." Polymer 54, 230 (2013)