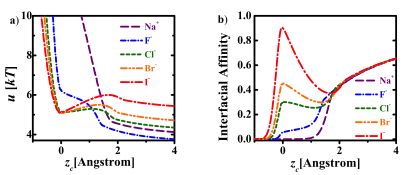Reports: ND553404-ND5: Effects of Ions on Interfacial Tension and Electrical Double Layer
Zhen-Gang Wang, California Institute of Technology
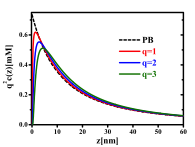
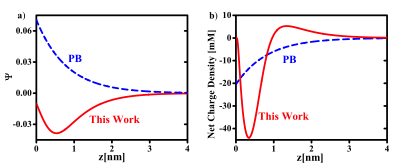
We proposed a new theory for the self-energy of salt ions across dielectric interfaces. By treating both the solvation and image force electrostatic forces as well as charge polarization induced by these forces in a consistent manner, we obtain a continuous self energy of an ion across the interface. Along with nonelectrostatic contributions, our theory enables, for the first time, a unified description of ions on both sides of the interface. Using intrinsic parameters of the ions, we predict the specific ion effect on the interfacial affinity of halogen anions at the water/air interface, and the strong adsorption of hydrophobic ions at the water/oil interface, in agreement with experiments and atomistic simulations. Our work constitutes a major progress towards resolving a long-standing debate on the key contributing factors for the specific ion effects. In particular, our work indicates that hydration forces and dispersion forces, while playing quantitative roles, are not essential for explaining the specific ion effects.
Impact on the Personnel PI: The study of the interfacial properties of salt solutions is a relatively new direction for the PI. The work supported by the ACS-PRF has allowed him to be recognized, within a relatively short period of time, as one of the key researchers in the field.
Graduate Student: The projects in this research have formed an excellent platform for the education and training of the graduate student. Working on resolving some long-standing puzzles and on revising standard textbook knowledge has been tremendously motivating for the student. Critically understanding the existing theories, finding the key missing ingredients in them and ways to improve them, and making predictions based on the new theories, have proved an enriching and rewarding experience for the student. In addition, he had the opportunity to present his work in the American Physical Society Meeting and the ACS Symposium for Colloid and Interface Science.