Reports: UR353427-UR3: Homogeneous Fischer-Tropsch Catalysts for the Conversion of Syngas into Higher Order Hydrocarbons
John D. Gilbertson, PhD, Western Washington University
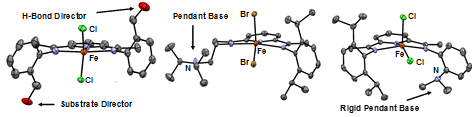
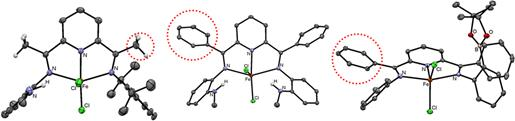
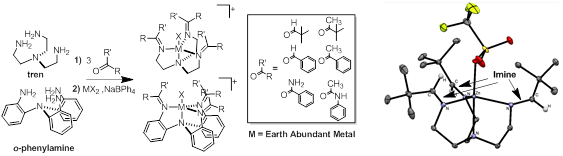
John D. Gilbertson, PhD, Western Washington University



Copyright © American Chemical Society