Reports: UNI1052586-UNI10: Incorporation of Calix[4]crowns Into Metal-Organic Frameworks for Hydrogen Storage
Xiangyang Lei, PhD, Lamar University
Progress
After we successfully synthesized one new organic linker bearing the bowl-shaped calix[4]crown moiety (1), during the second ACS-PRF grant year, we focused on the synthesis and characterization of its MOFs. The reaction of linker 1 and Zn(NO3)2 was carried out under different reaction conditions, including temperature, solvent, their molar ratio, and the usage of acid/base (Scheme 1). One MOF (2) has been obtained as single crystals, and its structure is under analysis by single crystal X-ray diffraction.
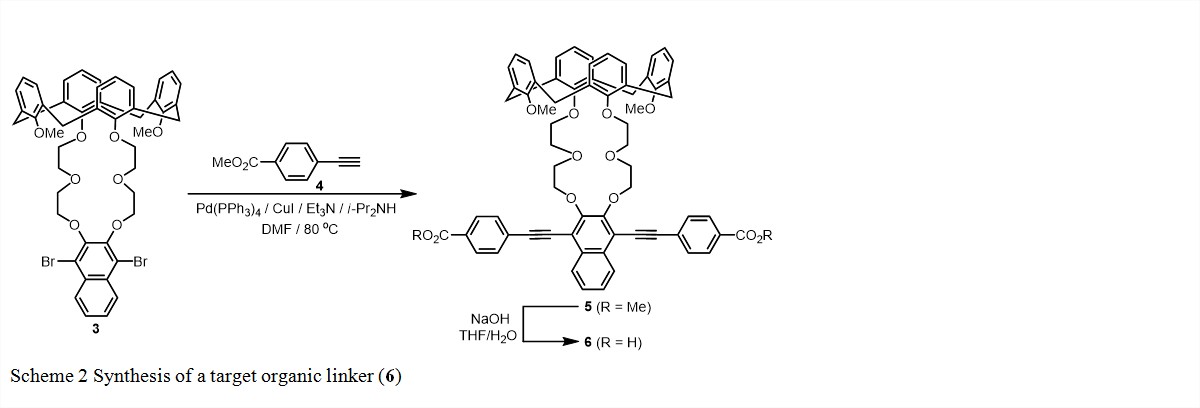
Meanwhile, we have been working on the synthesis of more organic linkers with different linker length. Following the synthetic routes developed in the first grant year, we synthesized 1,3-bridged calix[4]crown 3.1 Terminal alkyne 4 was synthesized from methyl 4-iodobenzoate in two steps via literature procedures.2 As shown in Scheme 2, the Sonogashira cross-coupling reaction of calix[4]crown 3 with alkyne 4 gave precursor 5, which was converted to linker 6by basic hydrolysis followed by acidic workup. The structures of the intermediates and the target organic linker have been characterized by 1H and 13C NMR.
Our future work includes the synthesis of organic linkers with different length of ether linkage and the synthesis of MOFs with linker 6. After the structure of MOF 2 is determined, the H2 and N2isotherms (adsorption and desorption) of the activated MOF will be measured at 77 K and 1 atm to determine its gravimetric and volumetric hydrogen uptake capacities, BET surface area, and pore volume, and confirm its permanent porosity.
Impact
This ACS-PRF grant provided summer salary support for two undergraduate students (Billy Cao and Jordan King) during summer 2013 and one undergraduate student (Mhd A. Abou Shama) during summer 2014. One graduate student, Anusha Alla, has been working on this project toward her M.S. degree. These students made great progress on this project and have gained valuable experience in literature search, organic synthesis, purification methods, instrumentation usage, and data analysis. Billy, Jordon and Anusha presented their research results at the annual poster competition in the Department of Chemistry and Biochemistry at Lamar University in October 2013, and Billy won first place in the undergraduate division. With the same research Billy also submitted a proposal to the 2014 Posters on the Hill Competition hosted by the Council on Undergraduate Research. The research experience helped the students develop problem-solving and critical-thinking skills that will facilitate their future studies and career.
References
1. (a) Gutsche, C. D.; Iqbal, M.; Stewart, D., Calixarenes. 18. Synthesis Procedures for p-tert-Butylcalix[4]arene. J. Org. Chem. 1986, 51, 742-745; (b) Gutsche, C. D.; Lin, L.-G., Calixarenes 12: The Synthesis of Functionalized Calixarenes. Tetrahedron 1986, 42, 1633-1640; (c) Loon, J.-D. V.; Arduini, A.; Coppi, L.; Verboom, W.; Pochini, A.; Ungaro, R.; Harkema, S.; Reinhoudt, D. N., Selective Functionalization of Calix[4]arenes at the Upper Rim. J. Org. Chem. 1990, 55, 5639-5646; (d) Bond, A. M.; Ghiggino, K. P.; Hogan, C. F.; Hutchison, J. A.; Langford, S. J.; Lygris, E.; Paddon-Row, M. N., Synthesis and Electrochemical Studies on a Crown Ether Bearing a Naphthoquinone Acceptor. Aust. J. Chem. 2001, 54, 735-738; (e) Gutsche, C. D., Calixarenes: An Introduction, 2nd Edition. The Royal Society of Chemistry: Cambridge, 2008; (f) Gutsche, C. D., Calixarenes Revisited. The Royal Society Chemistry: Cambridge, 1998; (g) Yamamoto, H.; Sakaki, T.; Shinkai, S., Regioselective Synthesis of 1,2- and 1,3- Bridged Calix[4]crowns. What are the Factors Controlling the Regioselectivity? Chem. Lett. 1994, 469-472.
2. Zhao, Y.-L.; Liu, L.; Zhang, W.; Sue, C.-H.; Li, Q.; Miljanić, O. Š.; Yaghi, O. M.; Stoddart, J. F., Rigid-Strut-Containing Crown Ethers and [2]Catenanes for Incorporation into Metal–Organic Frameworks. Chem. Eur. J. 2009, 15, 13356–13380.