Reports: DNI652494-DNI6: Dynamics and Kinetics of Carbon Dioxide in Nano-Porous Materials Environment from Quantum Molecular Dynamics Simulations
Yosuke Kanai, PhD, University of North Carolina (Chapel Hill)
|
|
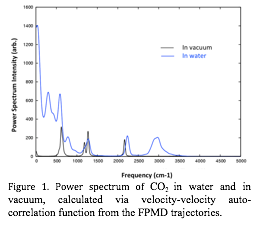
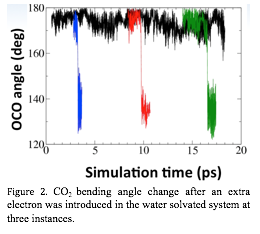
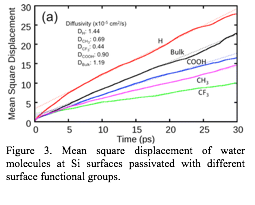
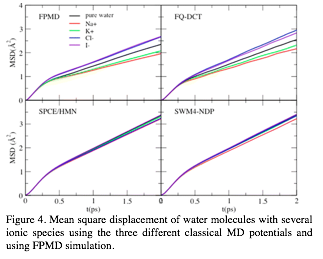
Yosuke Kanai, PhD, University of North Carolina (Chapel Hill)
|
|




Copyright © American Chemical Society