Reports: UR151961-UR1: Development of a Catalytic, Asymmetric Aza-Cope Rearrangement and Mannich Cyclization
Harriet A. Lindsay, Eastern Michigan University
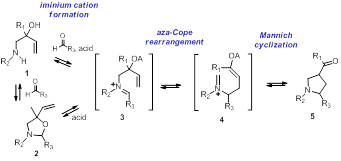
[1] Overman, L. E.; Kakimoto, M.-A. J. Am. Chem.
Soc. 1979, 101, 1310-1312.
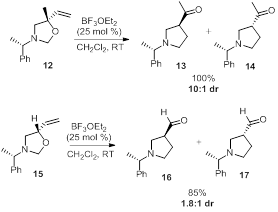
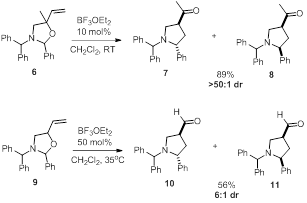
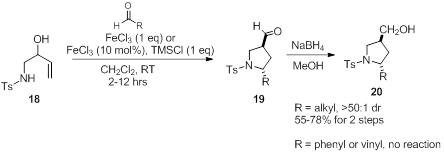
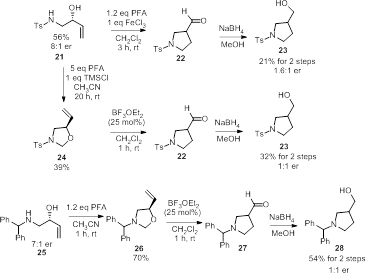
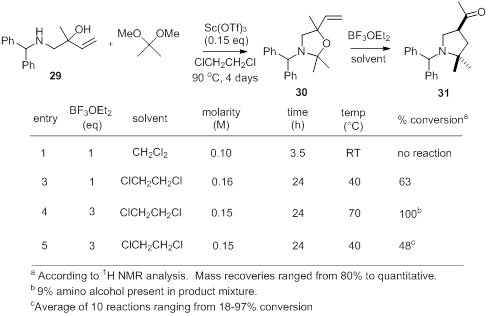
Harriet A. Lindsay, Eastern Michigan University

[1] Overman, L. E.; Kakimoto, M.-A. J. Am. Chem.
Soc. 1979, 101, 1310-1312.


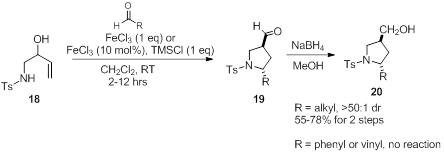


Copyright © American Chemical Society