Reports: ND453383-ND4: A New Spectroscopic Method to Assess the Oxidative Capacity of Iron Complexes in Catalytic Oxidation Reactions
Eike B. Bauer, PhD, University of Missouri - St. Louis
Cynthia M. Dupureur, PhD, University of Missouri - St. Louis
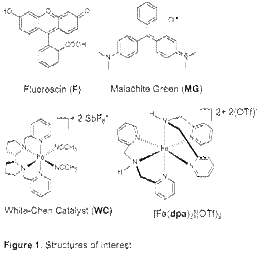
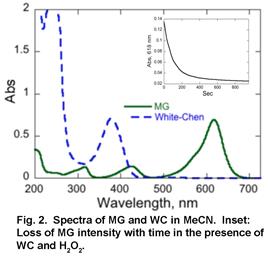
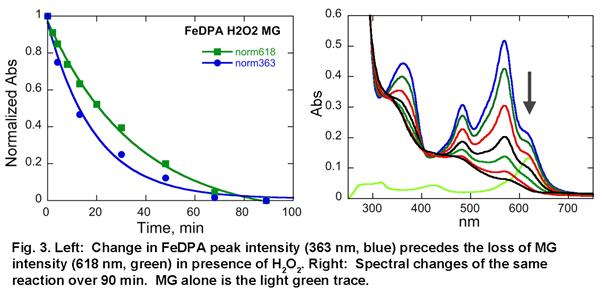
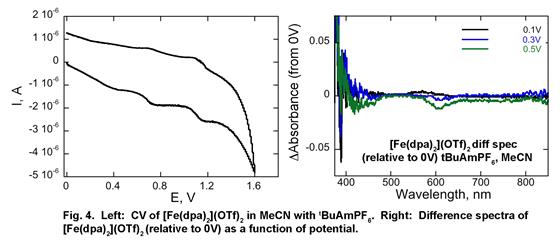
To normalize data obtained under different conditions for the purpose of comparison, we obtained first order rate constant for the reaction by collecting absorbance of the dye as a function of time at a range of metal complex concentration. The slope of this dependence is the first order rate constant with a unit of /time-metal complex concentration. Using this approach, we have already obtained preliminary 1st order rate constants for the oxidation of MG as catalyzed by WC catalyst in the presence of H2O2 (3.2e-2 μM-1min-1) and tBuOOH (6.2e-4 μM-1min-1). Similar measurements for [Fe(dpa)2]OTf2 are in progress.
During the first year of the grant period, we worked, as outlined above, on the development of the assay. Some issues such as choosing the correct reporter dye needed to be resolved. This required the direct expertise of Co-PI Dr. Dupureur. While students were involved where possible, their experiments were limited during this time, leading to underspending on the budget. However, more recently research students are synthesizing iron complexes and collecting spectral data on a much larger scale. The remaining funds will be applied to support this work and advance the project.
A manuscript to be submitted for publication describing the assay is currently in preparation. During the first year of the grant, we collected enough data to submit a NSF grant proposal, which happened on September 29, 2014.
References (1) Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E. K.; Prior, R. L.; Huang, D. Journal of agricultural and food chemistry 2002, 50, 2772.
(2) Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J. A.; Deemer, E. K. Journal of agricultural and food chemistry 2002, 50, 1815.
(3) Chen, F.; Ma, W.; He, J.; Zhao, J. J. Phys. Chem. A 2002, 106, 9485.