Reports: DNI152447-DNI1: Metal Catalyzed C-H Bond Activation and Allyl Addition: Development of New Transition Metal Catalysis for Practical and Uniquely Efficient Carbon-Carbon Bond Forming Reactions
Simon J. Meek, PhD, University of North Carolina (Chapel Hill)
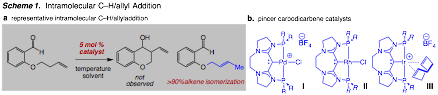
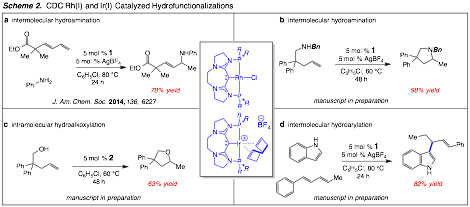
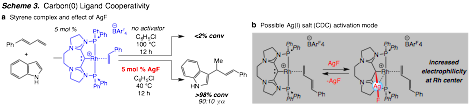
Copyright © American Chemical Society











