Reports: DNI1051865-DNI10: Understanding Ionic Liquid Aided Graphene Production by Exfoliation of Graphite
Gary A. Baker, PhD, University of Missouri, Columbia
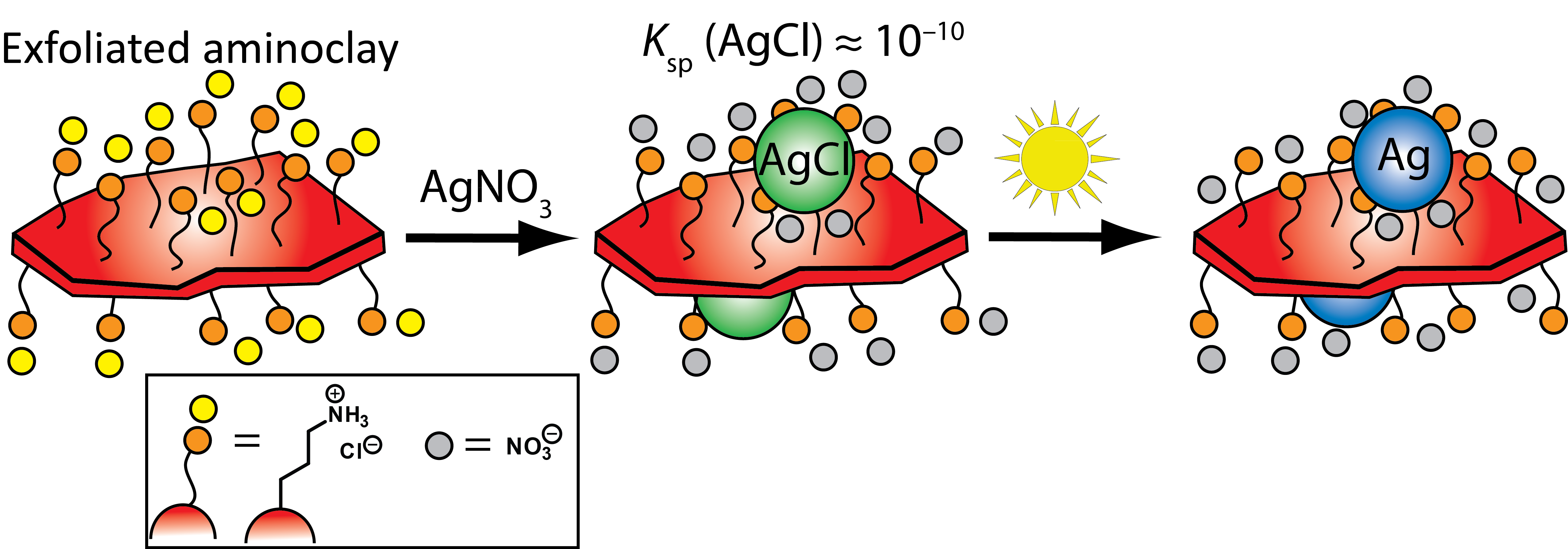
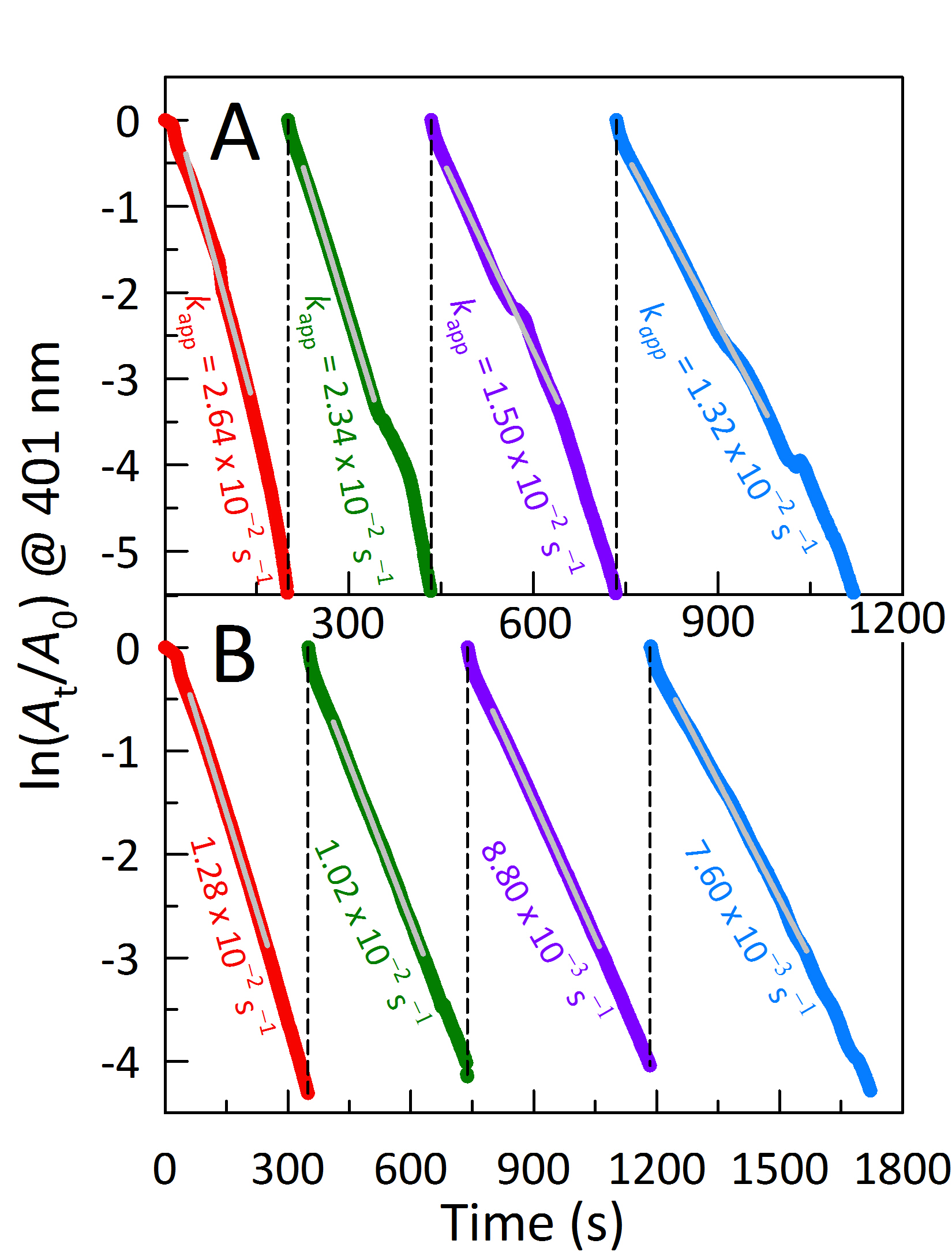
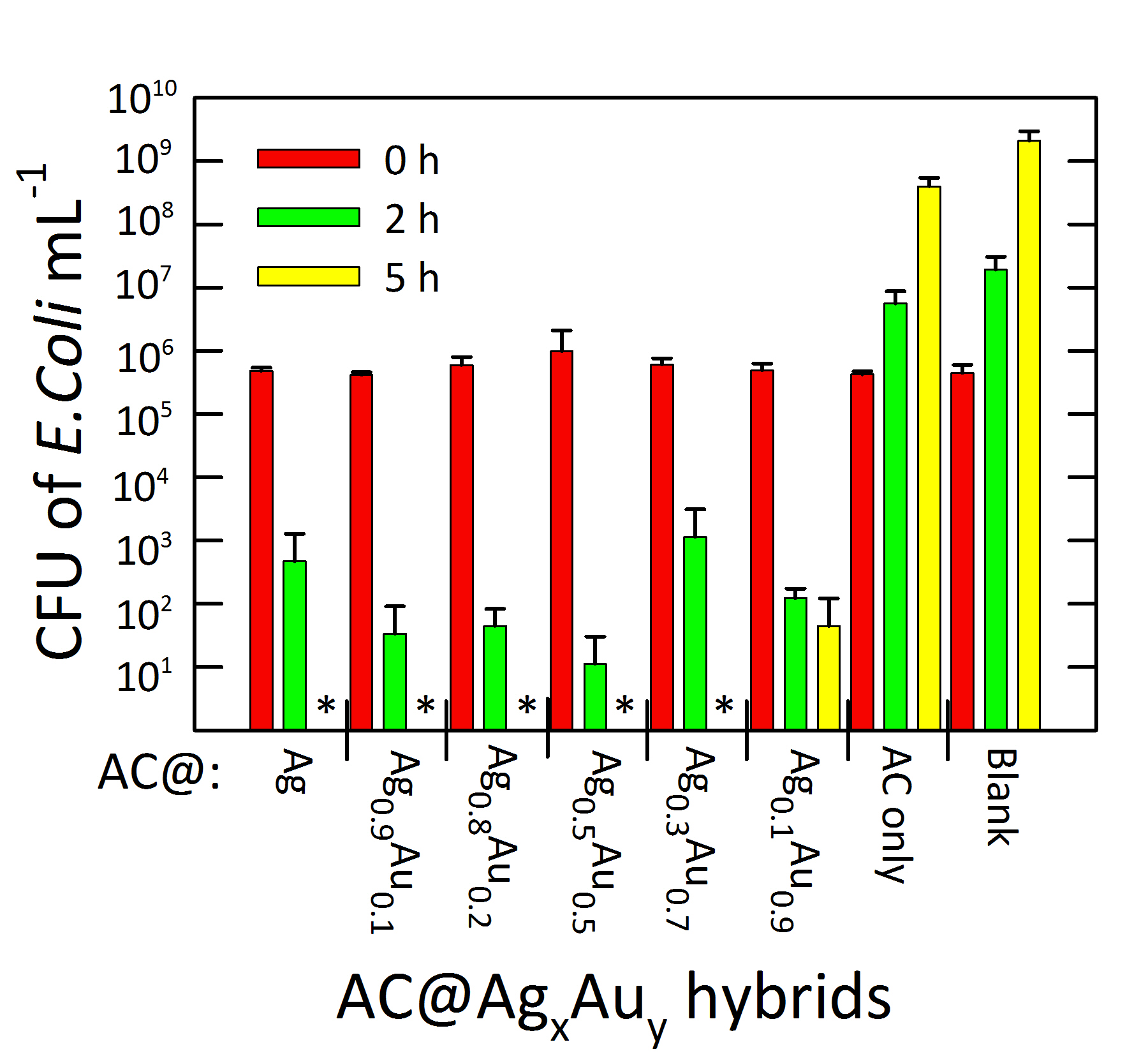
Gary A. Baker, PhD, University of Missouri, Columbia




Copyright © American Chemical Society