Reports: ND1052907-ND10: Large 'Molecular Panel' Based Metal-Organic Architectures for Gas Storage and Catalysis
Gellert Mezei, PhD, Western Michigan University
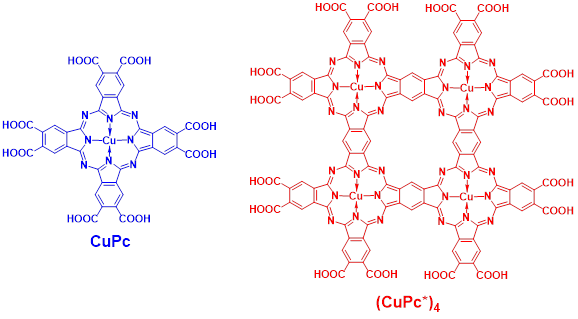
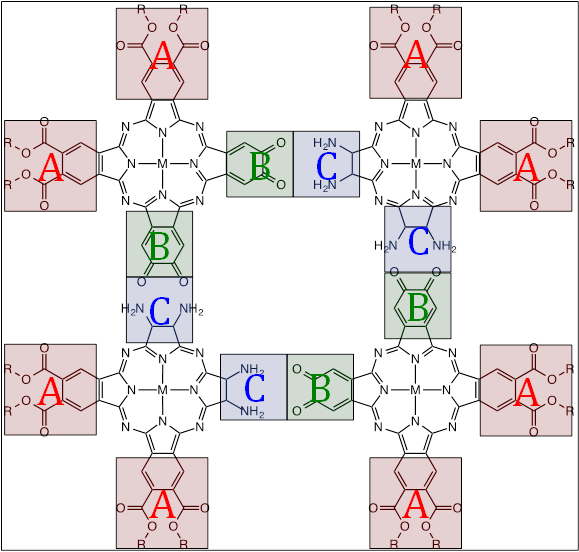
Figure 1. Structures of monomeric phthalocyanine octacarboxylic acid
CuPc and cyclic tetrameric phthalocyanine hexadecacarboxylic acid (CuPc*)4.
1.
Tetrakis-(N-((4-carboxy)phenyl)phthalimide) copper phthalocyanine
2.
Tetrakis-(N-((4-ethoxycarboxy)phenyl)phthalimide) copper phthalocyanine
3.
Tetrakis-(N-((3,5-dicarboxy)phenyl)phthalimide) copper phthalocyanine
4.
Tetrakis-(N-(4-pyridyl)phthalimide) copper chthalocyanine
5.
Tetrakis-(N-(p-tolyl)phthalimide) copper phthalocyanine
6.
Tetrakis-(N-(pyrazole-4-yl)phthalimide) copper phthalocyanine
7.
Tetrakis-(N-(anthracene-2-yl)phthalimide) copper phthalocyanine
8.
Tetrakis-(N-(pyrene-1-yl)phthalimide) copper phthalocyanine
9.
Tetrakis-(N-(4-(trifluoromethyl)-2H-chromene-2-one-7-yl)phthalimide) copper
phthalocyanine
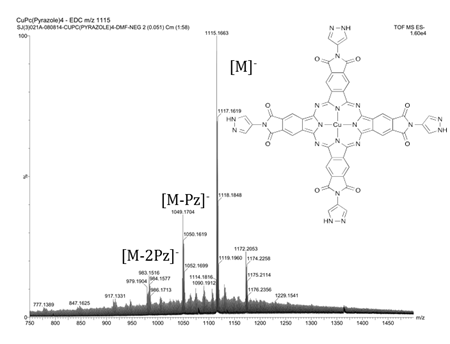
Figure 3. Electrospray
ionization mass spectrum of tetrakis-(N-(pyrazole-4-yl)phthalimide) copper
phthalocyanine.
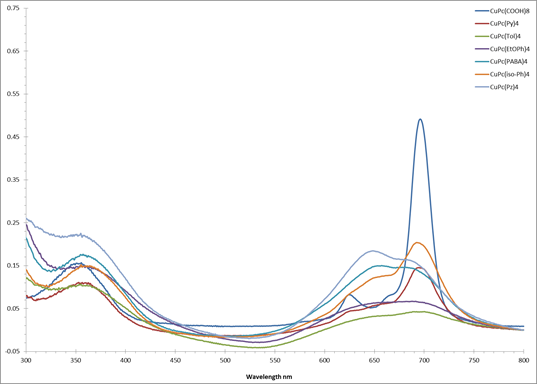
Figure 4. UV-vis spectra of variously
substituted copper phthalocyanine building blocks.
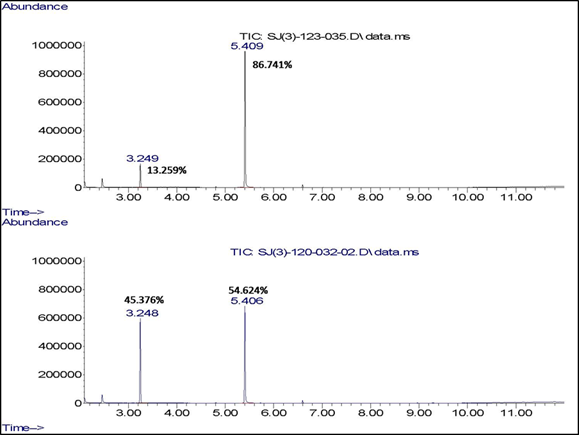
Figure 5. GC-MS analysis of
the reaction mixture obtained by the uncatalyzed (bottom) and 0.5 mol%
CuPc-catalyzed (top) oxidation of dibutylsulfide (at 3.25) to dibutylsulfoxide (at
5.41) by hydrogen peroxide after 2 hours.