Reports: UR552768-UR5: Selectivity Control in Aqueous Phenol Hydrogenation Using Palladium Nanocatalysts
Anderson L. Marsh, Lebanon Valley College
We have synthesized colloidal Pd nanocatalysts stabilized by polyvinylpyrrolidone (PVP) using solution based alcohol reduction methods published in the literature.1 Aqueous solutions of H2PdCl4 were prepared with 2.0 M HCl in 18 MO water. This H2PdCl4 solution, additional deionized water, ethanol, 40,000 g·mol-1 PVP, and 1 M HCl were refluxed in a round bottom flask for 3 h to produce a dark brown solution of colloidal PVP-capped Pd nanoparticles through the unbalanced reaction shown in equation 1:
Pd2+(aq) + PVP(aq) + CH3CH2OH(aq) → PVP-Pd0 + CH3CHO(aq) + 2 H+(aq) (1)
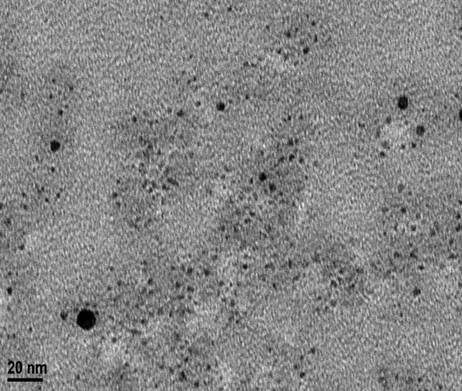
The concentration of ethanol in the refluxing mixture was 40% based on volume. A mole ratio of 20 to 1 for PVP to Pd, based on moles of PVP monomer units, was used in the synthesis. Particles synthesized with this route were in the 3 nm size range, determined by transmission electron microscopy (TEM) analysis. A representative TEM image is shown below in Figure 1.
Figure 1. TEM image of 3 nm Pd nanoparticles synthesized with 40,000 g·mol-1 PVP.
After washing and collection steps, these colloidal nanoparticles were redispered in deionized water to create 1.2 mM solutions based on moles of Pd. Using this route, we also prepared nanoparticles that were capped with 29,000 g·mol-1 or 55,000 g·mol-1 PVP while maintaining a constant mole ratio of 20:1 based on monomer unit and used the step-growth procedure to prepare particles of different sizes. In the first summer of the grant, we carried out reaction studies with these nanoparticles and varied the stirring rate of the reactor, along with the PVP molecular weight and reaction temperature. These experiments allowed us to determine the optimum stir rate to ensure the reaction is not under diffusion control.
During this past summer, we worked to prepare supported catalysts by depositing the colloidal nanoparticles on the surfaces of silica microspheres. We synthesized the silica microspheres via the Stöber method, in which tetraethyl orthosilicate is added to a solution of ethanol, water, and ammonium hydroxide and stirred over a 12 h period.2 To prepare the supported nanocatalysts, we used a simple adsorption method which involved stirring an aqueous mixture of the microsopheres and selected volumes of the PVP-capped nanoparticle solutions.3 The microsphere supported nanocatalysts have been characterized via TEM, as shown in Figure 2 below, to verify surface deposition and that palladium particle size is unchanged, as well as atomic absorption (AA) spectrometry to determine metal loading. At present the metal loadings have been in the 0.1 to 1% range, which are slightly lower than desired.
Figure 2. TEM image of silica microspheres loaded with 3 nm Pd nanoparticles capped with 40,000 g·mol-1 PVP.
Our reaction studies during the past summer were limited due to problems with the department’s gas chromatograph/mass spectrometer. However, the instrument is now up and running again and one of the students will be working on an independent study project this academic year to perform reactions with colloidal and supported nanoparticles. We will compare results from these experiments to those with a commercial Pd catalyst of similar loading.
During the first summer two students, a rising sophomore Josh Kauffman and a rising senior Jason Shay, were supported on the grant and worked a total of 10 weeks. At the end of the summer both students presented a poster at a regional scientific meeting and Jason presented at another regional meeting during the fall semester. While Jason planned to perform this research as part of a senior project, he was offered and accepted an internship with a pharmaceutical company at a local facility. His experience with analytical instrumentation on this project played a direct role in his being hired. Jason currently works in quality control for a different company at another local facility. During the first fall semester of the grant the PI also presented initial findings at the ACS meeting in Indianapolis. This past summer Josh, now a rising junior, and Nick Muench, a rising sophomore, were supported for a total of 10 weeks. Josh is continuing as part of his independent study. This project has allowed the PI to remain active in the field of nanocatalysis while providing research opportunities to continue his department’s tradition of hosting a summer research program.
References
1. Li, Y.; Boone, E.; El-Sayed, M. A. Size Effects of PVP-Pd Nanoparticles on the Catalytic Suzuki Reactions in Aqueous Solution. Langmuir 2002, 18, 4921-4925.
2. Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. Journal of Colloid and Interface Science 1968, 26, 62-69.
3. Gude, K.; Narayanan, R. Colloidal Supported Metal Nanoparticles (CSMNs) as Effective Nanocatalysts for Liquid-Phase Suzuki Cross-Coupling Reactions. Journal of Physical Chemistry C 2011, 115, 12716-12725.